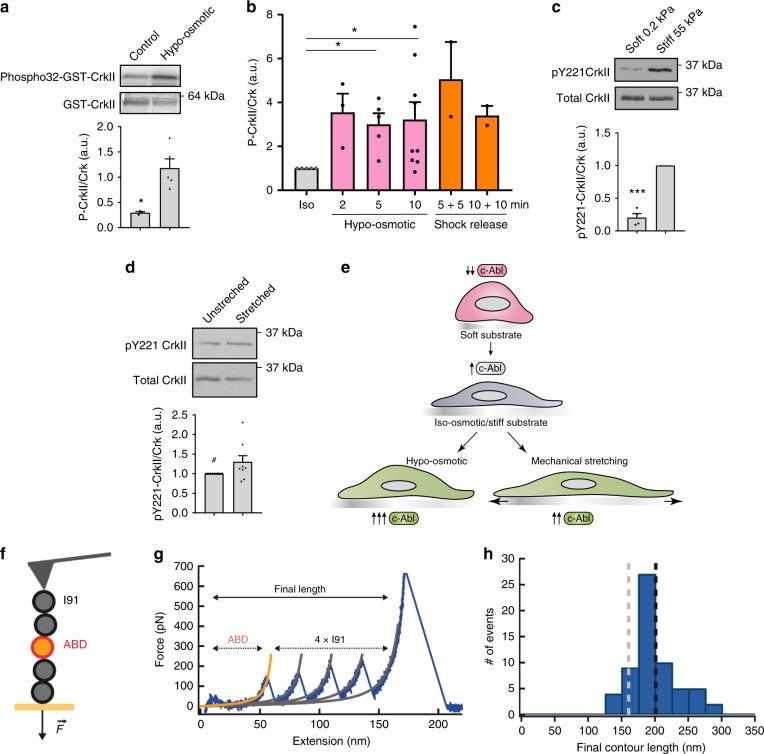
Fig. 7. C-Abl kinase is activated by mechanical forces and its actin-binding domain is mechanosensitive.
a The kinase activity of endogenous c-Abl is elevated after hypo-osmotic treatment. Endogenous c-Abl was immunoprecipitated from cells treated with iso-osmotic or hypo-osmotic medium (60 mOsm, 10 min) and in vitro kinase assay was performed using GST-CrkII as substrate. Phosphorylation (radiolabeled) and the total protein (Coomassie stained) signals are shown. N = 3 (control) and n = 5 (hypo-osmotic) biologically independent samples from 3 independent experiments. Statistical analysis with a two-tailed unpaired t test. *P < 0.05. b Kinetics of c-Abl activation upon osmotic swelling (30 mOsm) and after shock release is shown. Endogenous CrkII was immunoprecipitated from cells and pY221CrkII and CrkII was detected, normalized to iso-osmotic. From left to right, n = 5, 3, 5, 9, 2, 2, from biologically independent samples from the corresponding independent experiments. Statistical analysis with a two-tailed unpaired t test. *P < 0.05. c c-Abl kinase mechanosenses substrate rigidity. Endogenous CrkII was immunoprecipitated from cells grown in soft or stiff hydrogels and pY221CrkII and CrkII was detected. N = 4 biologically independent samples from 4 independent experiments. Statistical analysis with a two-tailed unpaired t test. *P < 0.05. d c-Abl kinase is activated by mechanical strain. Endogenous CrkII was immunoprecipitated from human fibroblasts unstretched and stretched for 10 min and pY221CrkII and CrkII was detected. #P = 0.08, n = 9 from biologically independent samples from 5 independent experiments. e Cartoon representing the different tensional states of a cell in culture and the activity of c-Abl depending on those tensional states. f Schematic representation of the heteropolyprotein (I91 ΔCys)2-ABD-(I91 ΔCys)2 used to characterize the mechanical properties of ABD. g Typical force-extension trace showing four unfolding peaks of the fingerprinting domain I91, which are preceded by a featureless extension which suggests that ABD unfolds at low forces. Solid lines show worm-like chain plots used to estimate contour lengths. h Final contour lengths of fingerprinted single-molecule force-extension traces. Dashed lines indicate theoretical final contour length values if ABD unfolds during the experiment (black) or remains folded (gray) are indicated. Data represent mean ± S.E.M.