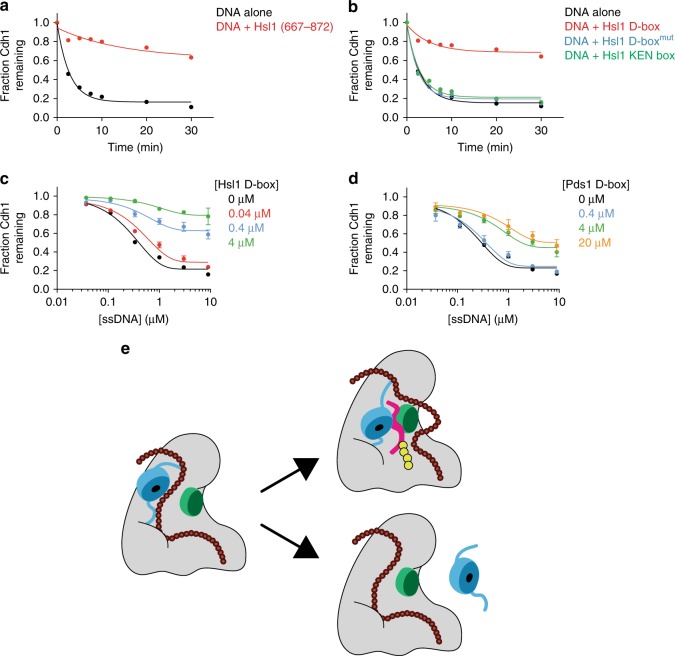
Fig. 6. D box-dependent substrate binding protects activator from dissociation.
a Cdh1 dissociation was measured with 1.5 μM 75-base ssDNA and 3 mM ATP, in the absence (black) or presence (red) of 4 μM purified Hsl1 fragment (aa 667–872). Uncropped autoradiographs and source data are provided in the Source Data file. b Cdh1 dissociation was measured in the presence of buffer (black), 4 μM Hsl1 D box peptide (red), Hsl1 D box peptide mutant (blue), or Hsl1 KEN box peptide (green). Reactions were performed in the presence of 1.5 μM 75mer ssDNA and 3 mM ATP. Uncropped autoradiographs and source data are provided in the Source Data file. c, d Cdh1 dissociation was measured in the presence of different concentrations of 75 mer ssDNA and D box peptides from c Hsl1 or d Pds1 (black 0 μM, red 0.04 μM, blue 0.4 μM, green 4 μM, orange 20 μM). Reactions were supplemented with 3 mM ATP. Data indicate means (±SEM) from two independent experiments. Uncropped autoradiographs and source data are provided in the Source Data file. e Model for dynamic regulation of APC/C activity by polyanions. Activator (blue) binding to the APC/C is mediated by binding motifs located on flexible N- and C-termini. Long polyanionic molecules (brown) interact at multiple sites on the APC/C, perhaps disrupting interactions between the activator termini and the APC/C. We speculate that one part of the polyanion chain interferes with productive substrate D box binding by pushing the activator WD40 domain away from Apc10 (green) and/or by directly interacting with Apc10. A high-affinity D box (red) overcomes this interference and links activator to Apc10, thereby promoting activator binding and substrate ubiquitylation (yellow).