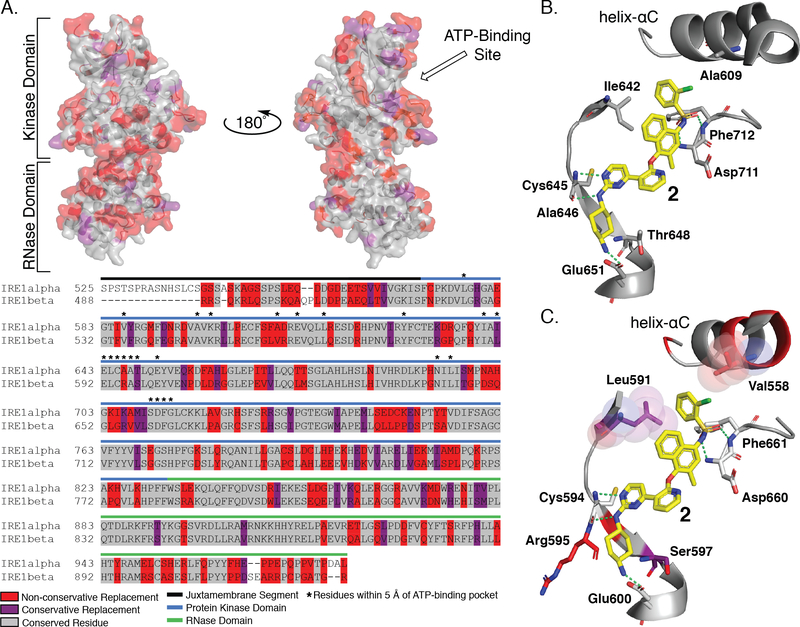
Figure 6. Sequence Alignment and Structural Comparison of IRE1α and IRE1β ATP-Binding Sites.
A. Sequence alignment of IRE1α and IRE1β (top) Sequence comparison between IRE1α and IRE1β mapped onto the crystal structure of IRE1α (PDB: 4U6R). Residues that are conserved are shown in gray, residues that have a conservative replacement are shown in purple, and residues with non-conservative replacements are shown in red. (bottom) Sequence alignment of IRE1α and IRE1β shows 80% sequence identity of the kinase domain and 61% sequence identity of the RNase domain. B. Interactions between 2, a close structural analog of KIRA 1, and the ATP-binding site of IRE1α. Compound 2 is shown as yellow sticks, key interacting residues are shown as gray sticks, and interactions are denoted with green dashed lines. C. Hypothesized binding mode of KIRA 2 by mapping of non-identical residues of IRE1β onto the structure of 4U6R. Conservative replacement non-identical residues are shown as purple sticks, non-conservative non-identical residues are shown as red sticks. Residue numbering is for IRE1β.