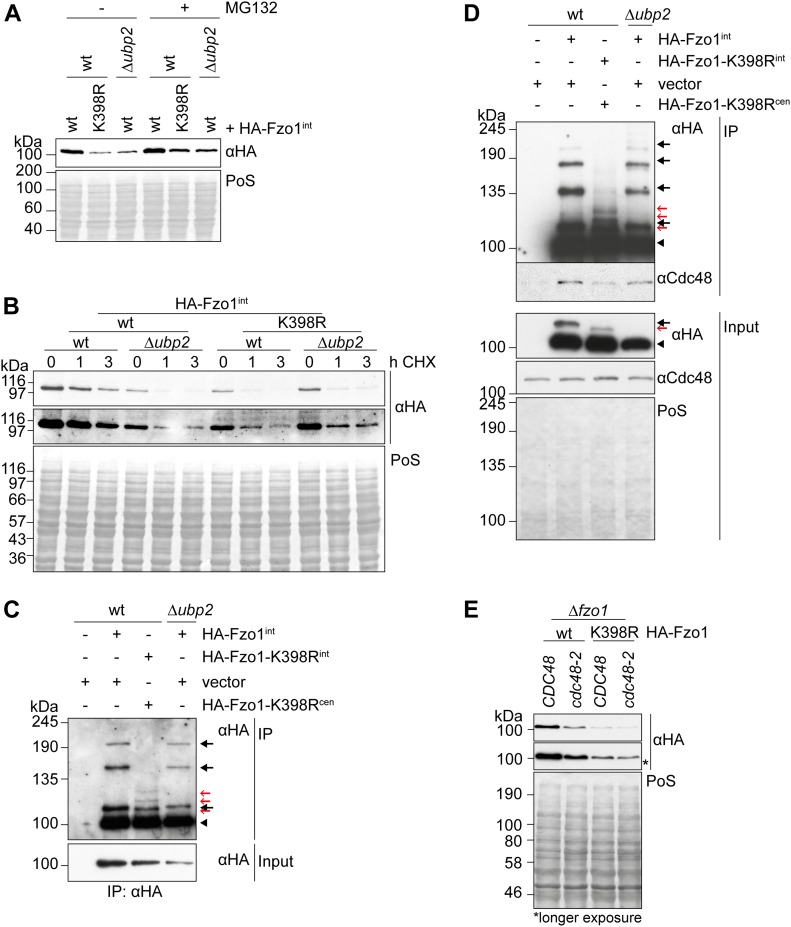
Figure 3. Loss of wild-type ubiquitylation renders Fzo1 insensitive to Ubp2 and Cdc48.
(A) Effect of proteasome inhibition and UBP2 deletion in the steady state levels of Fzo1 and Fzo1K398R. The proteasome was inhibited as described in Fig 2A in ∆pdr5 (indicated as wt) or in ∆pdr5 ∆ubp2 (indicated as ∆ubp2) cells, expressing genomically integrated HA-tagged Fzo1 or Fzo1K398R. Total cellular extracts were analysed by SDS–PAGE and Western blot using an HA-specific antibody. (B) Stability of genomically HA-tagged Fzo1 or Fzo1K398R in wt or ∆ubp2 cells. The turnover of genomically HA-tagged Fzo1 (wt) or Fzo1K398R (wt) in wt or ∆ubp2 cells was assessed as in Fig 2A. The samples were analysed by SDS–PAGE and Western blot using an antibody against HA. (C) Comparison of the ubiquitylation pattern of Fzo1 upon UBP2 deletion or Fzo1-K398R mutation. Crude mitochondrial extracts from wt cells (expressing genomically HA-tagged Fzo1 or Fzo1K398R) or ∆ubp2 cells (expressing genomically HA-tagged Fzo1) were solubilized, subjected to HA-immunoprecipitation, and analysed by SDS–PAGE and Western blot using an HA-specific antibody. Forms of Fzo1 are indicated as in Fig 1A. (D) Analysis of Cdc48 immunoprecipitation with genomically HA-tagged Fzo1 or Fzo1K398R in wt or ∆ubp2 cells. (C) Crude mitochondrial extracts from cells as in (C) (but grown at 37°C) were solubilized, subjected to co-immunoprecipitation, and analysed by SDS–PAGE and Western blot using HA- and Cdc48-specific antibodies. Forms of Fzo1 are indicated as in Fig 1A. (E) Steady state levels of HA-tagged Fzo1 and FzoK398R in wt and cdc48-2 cells. Total cellular extracts of wt or cdc48-2 cells, ectopically expressing HA-tagged Fzo1 or Fzo1K398R, were analysed by SDS–PAGE and Western blot, using an HA-specific antibody. IP, immunoprecipitation; PoS, Ponceau S.