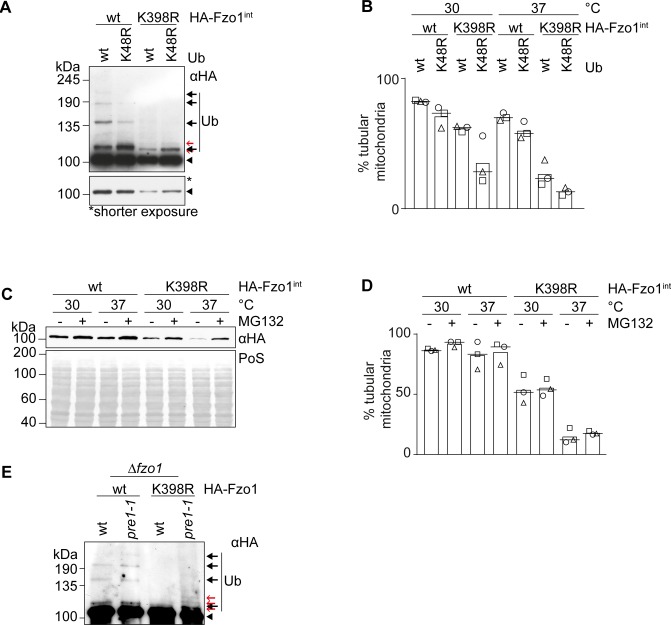
Figure S4. Proteasomal inhibition does not rescue fusion in the Fzo1-K398R mutant strain.
(A) Analysis of the ubiquitin linkage type of Fzo1 and Fzo1K398R. Crude mitochondrial extracts from cells expressing endogenously HA-tagged Fzo1 (wt) or Fzo1K398R (K398R) and ectopically expressing either wt ubiquitin (Ub wt) or ubiquitin with a K48R mutation (Ub K48R) were solubilized, subjected to HA-immunoprecipitation, and analysed by SDS–PAGE and Western blot, using an HA-specific antibody. Forms of Fzo1 are indicated as in Fig 1A. (A, B) Mitochondrial morphology of cells as described in (A) and performed as described in Fig 1B. (C) Rescue of HA-Fzo1K398R steady state levels by inhibition of proteasomal degradation. Exponentially growing cells defective for the multidrug exporter Pdr5 (Δpdr5) expressing endogenously HA-tagged Fzo1 (wt) or Fzo1K398R (K398R) were treated with the proteasomal inhibitor MG132 (or DMSO as a control) for 1 h. Total cellular extracts were analysed by SDS–PAGE and Western blot using an HA-specific antibody. (C, D) Mitochondrial morphology of cells as described in (C) and performed as described in Fig 1B. (E) Ubiquitylation of HA-tagged Fzo1 or Fzo1K398R in proteasomal mutant pre1-1. Crude mitochondrial extracts from wt or pre1-1 cells expressing HA-Fzo1 (wt) and HA-Fzo1K398R (K398R) were solubilized, subjected to HA-immunoprecipitation, and analysed by SDS–PAGE and Western blot using an HA-specific antibody. Forms of Fzo1 are indicated as in Fig 1A. PoS, Ponceau S; Ub, ubiquitin.