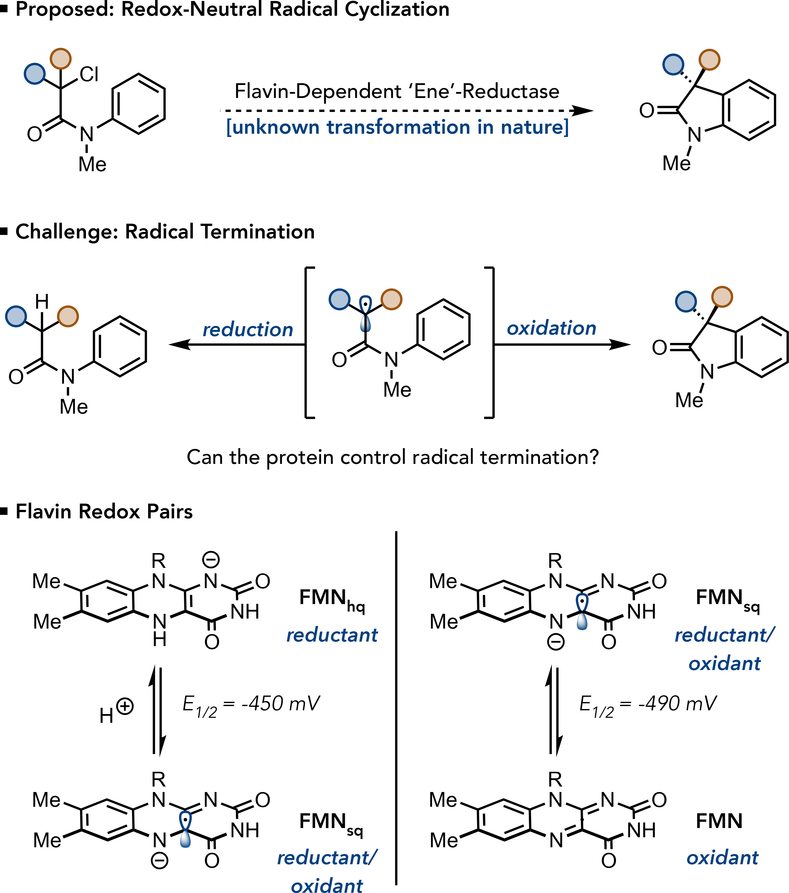
Fig. 1. Strategies and Challenges in Using ‘Ene’-Reductases for Redox-Neutral Radical Cyclizations.
(a) The desired redox-neutral radical cyclization to prepare oxindoles from α-haloamides, a transformation currently unknown in nature (b) The central challenge to this reactivity is identifying a flavin redox couple that will favor cyclization over reduction. (c) The viable flavin redox pairs for redox-neutral cyclizations. If FMNhq is responsible for reducing the starting material, FMNsq will need to serve as an oxidant for the desired transformation. FMNsq can also function as a reductant, providing access to an undesired reductive mechanism. However, if FMNsq is used to reduce the starting material, FMN is formed in the active site, which can only function as an oxidant.