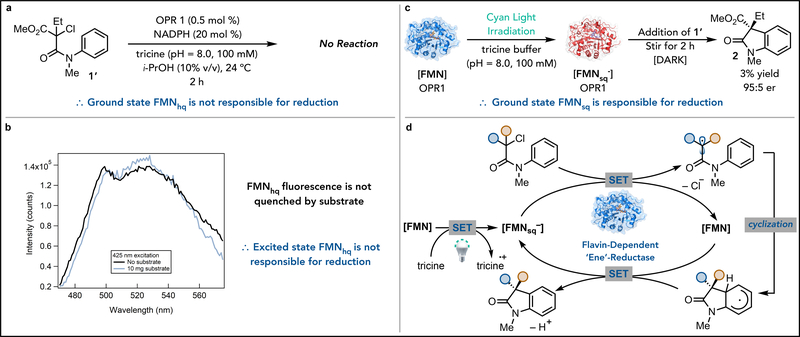
Fig. 2. Studies to determine the mechanism of oxindole formation.
(A) This experiments demonstrates that ground state FMNhq is not able to initiate the radical cyclization. (B) The fluorescence spectra indicates that the excited state can be accessed, however, this state is not quenched by the substrate, indicating that FMNhq* is not responsible for initiating the reaction. (C) In this experiment, oxidized OPR1 is photoreduced with cyan light and tricine buffer to partially reduce FMN to FMNsq− (as determined by EPR). Then substrate 1’ is introduced to the enzyme in the absence of light. Oxindole is formed under these conditions indicating that gound state FMNsq− is responsible for initiating the reaction. (D) This represents a proposed mechanism where light and tricine buffer are responsible for reducing FMN to FMNsq−, which can reduce the substrate to generate an α-acyl radical and FMN. Cyclization of the radical generates a reducing vinylogous amido radical which can be oxidized by FMN to form product and regenerate FMNsq−.