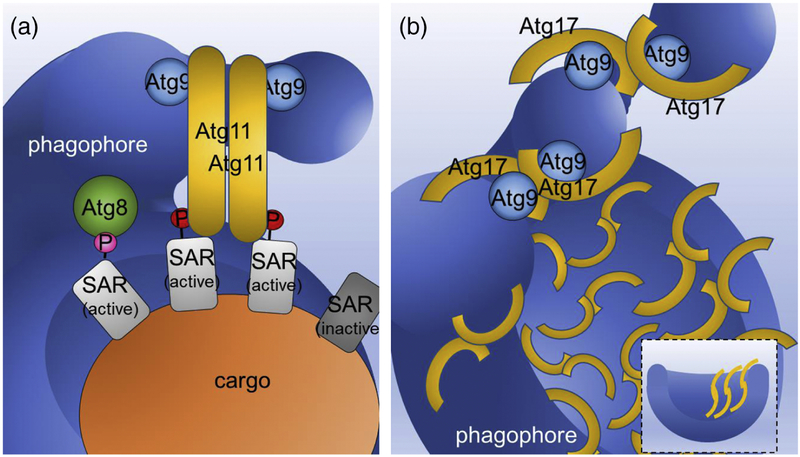
Figure 3. Comparison of proposed mechanisms of scaffolding the cup-shaped double membrane of the autophagosome by Atg11 and Atg17.
A. Proposed model for the role of Atg11 in membrane tethering at the selective PAS. Only SARs (light gray) activated by phosphorylation at their Atg11-binding regions (A11BRs) (A11BR phosphorylation is indicated by a red circle labelled and the pink circle labelled P shows AIM phosphorylation) interact with Atg11. Binding to activated SAR stimulates Atg11 dimerization in a parallel manner. Atg11 also interacts with Atg9-vesicles and Atg9-decorated phagophore membranes. In this state, Atg9-vesicles are brought into close physical proximity to the phagophore, which along with other components of the autophagy machinery (not shown for simplicity) initiates their tethering and drives their fusion. Atg8 binds to SARs phosphorylated at their AIMs. B. Proposed model for the role of Atg17 in membrane tethering at the non-selective PAS. Note that non-selective cargo is not shown for simplicity. Due to the antiparallel dimerization of Atg17, this protein, together with the Atg31-Atg29 subcomplex (not shown for simplicity) forms the Atg17–Atg31–Atg29 double crescent. In induced conditions, Atg17 dimer formation is activated, leading to the subsequent binding of Atg9-vesicles to the double crescents and their localization next to one another. Next, as described for Atg11 tethering, with the assistance of the autophagy machinery (not shown for simplicity), Atg9-vesicle tethering and subsequent fusion is initiated. Note that computational integration of coarse-grained protein complexes into a model of membrane shape dynamics[26] revealed that the Atg17 dimer also matches the profile of the bowl-shaped phagophore membrane and therefore does not need the cargo for scaffolding and shaping the autophagosome (inset at bottom right). For more information regarding Atg17 scaffolding, the cup-shaped double membrane in autophagy and how multiple Atg17 dimers cooperate to remove the barriers for the transition from tubular to disk shapes, and then to phagophore shapes, see Ref. 26.