Fig. 4.
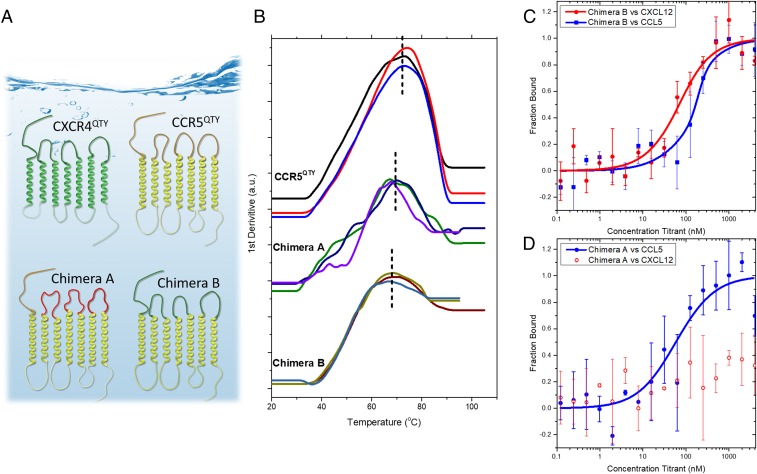
Design chimeric QTY receptors. (A) Schematic of the chimera design. CXCR4QTY is green. CCR5QTY’s 7TM regions (yellow) and IC loops were chosen as the backbone of the design (protein sequences are in SI Appendix). In Chimera A (Bottom Left) (CCR5QTY-N-3(GS)n) the 3 EC loops of CCR5QTY were replaced by GS linker sequence (red) with the same length (Upper, yellow loops), but CCR5QTY N terminus (red line) was unchanged. In Chimera B (Bottom Right) (CCR5QTY-7TM: CXCR4QTY-N,3ECL), the N terminus and EC loops of CCR5QTY were replaced by N terminus and 3 EC loops of CXCR4QTY (green N-terminal and 3 green loops). (B) Three independent Tm measurements by NanoDSF to evaluate the thermostability. CCR5QTY: 73.3 ± 0.9 °C, Chimera A: 68.2 ± 1.8 °C, Chimera B: 68.3 ± 1.8 °C. (C and D) MST ligand-binding measurement for Chimera A and Chimera B. (C) Chimera B binds both CXCL12 and CCL5 with affinity of 54.7 ± 19.6 nM and 150 ± 45 nM, respectively. (D) Chimera A binds CCL5 with affinity of 64.4 ± 40.8 nM. These results suggest that the N terminus and 3 EC loops are the most crucial regions for the ligand-binding activities of these chemokine receptors.