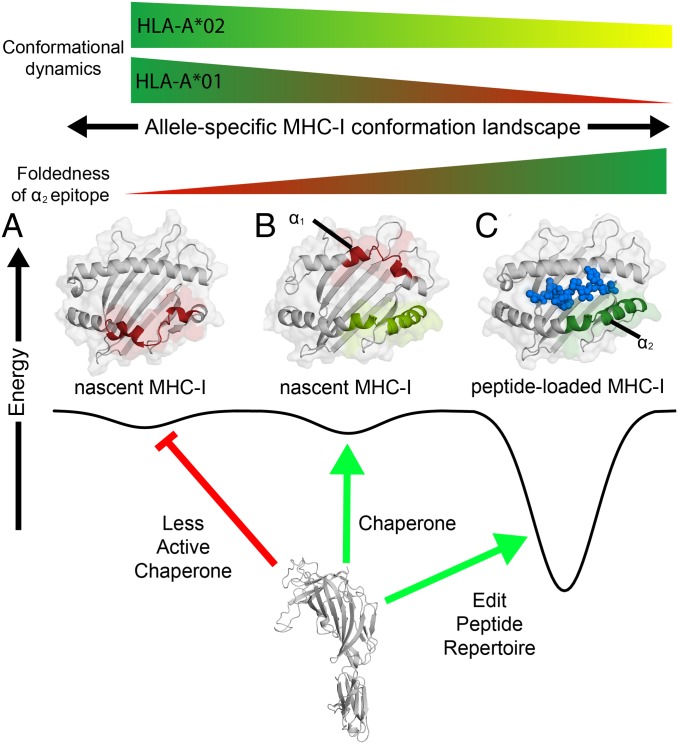
Fig. 7.
Chaperone recognition of a dynamic MHC-I conformational landscape. Conceptual example of the interaction between the chaperone TAPBPR and different MHC-I conformations of varying energetic and structural features. The vertical axis is free energy and the horizontal axis represents the conformational landscape of the MHC-I, which is influenced by specific polymorphisms in the MHC-I groove (α1/α2) and α3 domains. (A) TAPBPR does not associate with an MHC-I state comprising a misfolded α2 domain (red). (B) In the chaperoning function, TAPBPR interacts with nascent MHC-I conformations consisting of a folded α2 domain (light green) with an oxidized disulfide bond between the conserved Cys-101/164, even if the α1 domain remains in a misfolded state (red). (C) As a peptide editor, TAPBPR recognizes properly conformed molecules loaded with peptides toward exchange of the bound peptide cargo, for those alleles that exhibit μs-ms time scale conformational dynamics at the α2–1 helix (green). The peptide in the properly conformed pMHC-I state is shown as blue spheres.