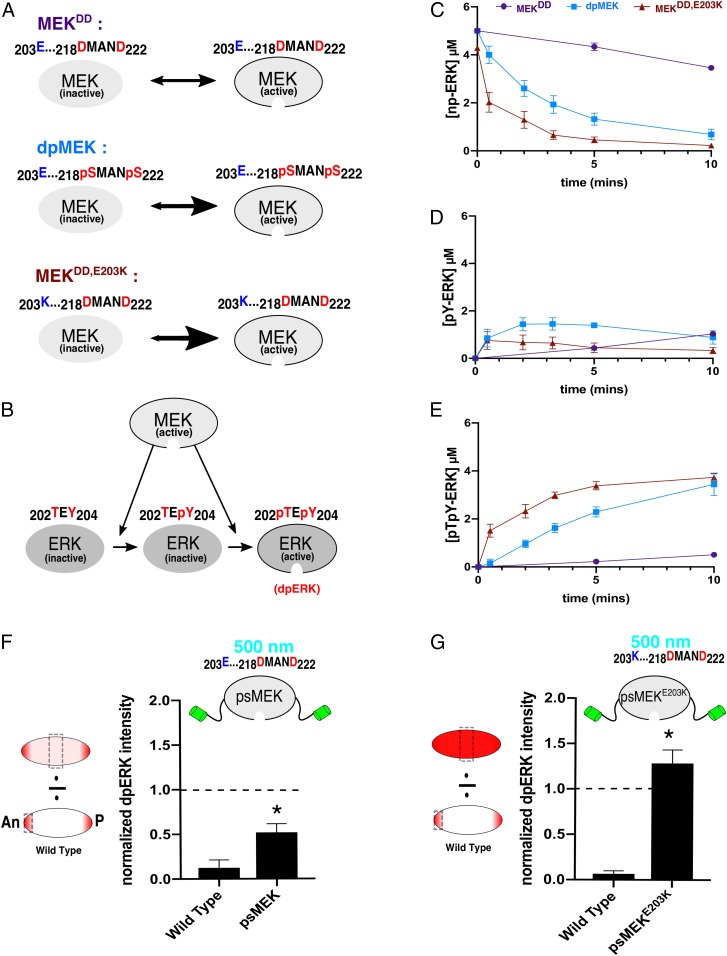
Fig. 2.
Enhancing MEK activity with destabilizing mutations. (A) Phosphomimetic MEK (MEKDD) is predicted to be significantly less active than dpMEK. Destabilizing MEKDD with a distal E203K substitution (blue) creates a different MEK variant (MEKDD,E203K) that we predict will exhibit increased activity. (B) Active MEK catalyzes dual phosphorylation of ERK. (C) Data from in vitro kinase assays showing kinetics of unphosphorylated ERK (np-ERK) levels in the presence of phosphomimetic (purple, MEKDD), phosphorylated (light blue, dpMEK), and phosphomimetic MEK with E203K (dark red, MEKDD,E203K). Data are based on n = 5 for MEKDD and dpMEK and n = 6 for MEKDD,E203K at all time points. (D) Time courses of the monophosphorylated ERK (pY-ERK) levels for 3 different MEK variants. (E) Time courses of the dually phosphorylated ERK (dpERK, pTpY-ERK) levels for 3 different MEK variants. (F) Quantifications of confocal images of immunofluorescence stainings for dpERK in 3-h-old Drosophila embryos. The dpERK intensities in the middle of the embryo (50% embryo length) are presented for WT and psMEK-expressing embryos activated with 500 nm light. All measurements reflect the dpERK levels normalized to the maximum dpERK intensity in the WT control embryos (at the anterior pole). The dashed line drawn at 1.0 represents the maximum dpERK intensity in WT embryos. NWT = 17 and NpsMEK = 25. (G) The same quantifications described for embryos expressing psMEK with an additional E203K mutation, denoted as psMEKE203K. NWT = 18 and NpsMEKE203K = 15. In F and G, *P < 0.05.