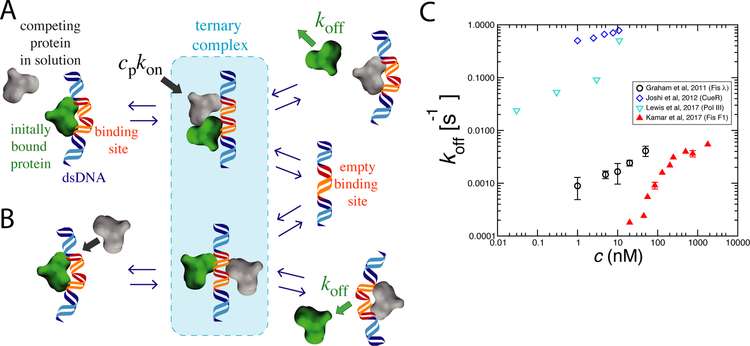
Figure 1: Kinetic pathways for facilitated dissociation (FD) and experimental data.
A) Competing protein from solution (gray) binds to partially exposed DNA site arising from thermal fluctuation of previously bound protein (green). In the ternary complex, each protein is weakly bound, which leads to dissociation of one or both proteins.
B) Within the ternary complex, proteins can compete may interfere with one another’s binding via allostery through the substrate as well as via sterically blocking one anothers’ interactions with the DNA.
C) Experimental data showing protein off-rate increasing with bulk protein concentration for a few DNA-binding proteins (see text for details). In each case the off-rate increases with solution concentration of that protein, the hallmark of facilitated dissociation.