Abstract
Large osteochondral lesions of the knee in young patients continue to be a challenge for orthopaedic surgeons and the focus of continual research. This is particularly true if the injury is a consequence of a dysplastic trochlea and involves both articular surfaces of the biomechanically complex patellofemoral joint. To obtain a healthy and congruent patellofemoral joint, the use of a bipolar fresh osteochondral allograft transplantation of the patella and trochlea is one of the few options to biologically treat these injuries. This would achieve a replacement of the entire articular surface of the patellofemoral joint with a high number of viable chondrocytes and respect the unique structural characteristics of the cartilage. The aim of this study was to obtain symptomatic and functional improvements while delaying the timing of prosthetic surgery. We present a reproducible although demanding surgical technique to perform a bipolar fresh osteochondral allograft transplantation of the patella and trochlea.
Treatment of osteochondral knee injuries in young and active patients continues to be a challenge for the orthopaedic surgeon and the focus of continual research. Chondral lesions on the patella and trochlea are particularly difficult to manage because of their biomechanical characteristics, shape, and size.1, 2, 3, 4
The goal of surgical treatment is to correct the biomechanical alterations and anatomic deformities and to treat the chondral lesions in accordance with their location, extension, and depth.1, 2 The treatment options for patellofemoral chondral lesions range from simple arthroscopic debridement and microfracture to more invasive procedures5 such as osteochondral autograft transplantation,6 osteochondral allograft (OCA) transplantation, autologous chondrocyte implantation,7 matrix-induced autologous chondrocyte implantation,8 and patellofemoral arthroplasty.9
When the defect is focal and localized in an area with good stability, thus with better integration, osteochondral grafts can be of a relatively small size. In the case of large or multifocal lesions, particularly with a highly dysplastic trochlea, full osteochondral resurfacing of the patellofemoral joint would not only approach the cartilage defect but also address the cause of the injury.10
Having viable chondrocytes guarantees the viability of the graft after transplantation.11 Among all the different tissue bank storage techniques, fresh allografts are the only ones that provide for the long-term viability of the chondrocytes.12 However, the allografts must be transplanted within 14 to 28 days after procurement for maximum chondrocyte viability.13
Most authors have reported osteochondral transplantation of the trochlea using large plugs. Even so, a dysplastic trochlea must be addressed differently. A trochleoplasty is performed for patellofemoral instability. However, it is contraindicated in the presence of large cartilage defects. Therefore, a combination of a large cartilage injury and a dysplastic trochlea can only be biologically treated by a total resurfacing osteochondral transplantation.
The purpose of this technical note is to present a reproducible technique to address large osteochondral defects of the patellofemoral joint with a fresh OCA transplantation in the presence of a dysplastic trochlea. This technique aims to reduce the symptoms and to delay the need for a prosthetic implant in an otherwise healthy young patient.
Indication
Fresh osteochondral transplantation of both complete articular surfaces of the patella and trochlea is indicated in subjects younger than 50 years. They will have an extensive cartilage or osteochondral lesion in the patella and trochlea (kissing lesion) with Dejour type C or D trochlear dysplasia that cannot be treated with other, less aggressive techniques. Only patients with severe chronic pain that limits their daily activities and without reasonable improvement with conservative treatment are candidates for this option.
The main exclusion criteria are advanced osteoarthritis of other compartments of the knee and general conditions such as infections, tumors, locally aggressive rheumatic disease, diabetes, and vasculitis. Relative contraindications are a body mass index greater than 30 and age older than 50 years, although a clear age cutoff has not yet been defined. Smoking must be stopped 30 days prior to the surgical procedure and abstained from for at least 6 months after the operation.
Preoperative Study
All patients undergo the following radiologic evaluation:
-
•
A long-standing radiograph, with lateral and axial views of both knees
-
•
Magnetic resonance imaging to assess the chondral or subchondral status of the patellofemoral joint, as well as to look for any potential concomitant injuries
-
•
A computed tomography scan to provide the most accurate information on bone loss and allow for measurement of the defect and disorders of the patellofemoral joint
Fresh Allograft Harvesting
The local authorized tissue bank supplied the allografts and performed the preoperative graft processing. The fresh OCA should be obtained from donors younger than 45 years. The limit for harvesting fresh grafts is within the first 12 hours of death. However, the period can be extended by an extra 12 hours if the donor's body is kept refrigerated at 4°C for the first 4 to 6 hours after death.
The harvested OCA is placed into transport medium (lactated Ringer solution) and preserved refrigerated between 4°C and 8°C. Once in the local authorized tissue bank, preparation and cleaning of the grafts are performed in a class A clean room. This consists of the evaluation of the cartilage surface, removal of the soft tissue and periosteum, shaping of the grafts, and high-pressure pulsatile lavage irrigation with sterile saline solution. The finals grafts are subjected to a decontamination process consisting of dry centrifugation, followed by centrifugation with sterile phosphate-buffered saline solution. Microbiological tests are then carried out on the grafts and on the last wash solution. Finally, the allografts are submerged in a preservative solution consisting of lactated Ringer solution and an antibiotic cocktail that includes amphotericin (125 μg/mL), tobramycin (3 mg/mL), vancomycin (50 mg/mL), and co-trimoxazole (160 mg/mL). After 5 days, new microbiological tests are performed on the allografts and on the preservative solution. From its arrival at the tissue bank until the time of implantation in the patient, the allograft is kept refrigerated between 4°C and 8°C.
Currently, the estimated risk of infectious disease transmission is 1 case in 420,000 donations for hepatitis C virus, 1 in 175,000 for human immunodeficiency virus, and 1 in 100,000 for hepatitis B virus.14 Allograft sizing is performed in accordance with a preoperative computed tomography scan (both of the patient and of the graft) and anthropometric agreement between the donor and recipient.
Surgical Technique
Positioning
The patient is placed in the supine position with a support for the foot and a lateral support for the thigh to maintain the knee at around 45° of flexion. The contralateral limb is placed in full extension.
Donor Graft Preparation
The distal femoral and patellar grafts are first warmed up to room temperature and then inspected to ensure the absence of macroscopic damage. The articular side of the patella is resected using a standard cutting patellar guide such as that used in total knee arthroplasty (Fig 1). Only 6 to 8 mm of the subchondral bone tissue is resected with the guide. This step is of utmost importance because the thicker the bone tissue of the graft, the greater the possibility of an immunoreaction and bone resorption. In addition, osteochondral integration is only achieved because of the creeping substitution phenomenon in the first 8- to 12-mm layer of bone tissue.15 It is crucial to minimize any potential immunologic reaction by removing all the remaining soft tissue and by washing with a 6-L high-pressure pulsatile lavage irrigation system inside a proper tube-like recipient. Lavage is performed for at least 15 minutes. A mark is made at the proximal and lateral part of the patellar graft with a sterile skin marker to aid in its proper placement later in the recipient area.
Fig 1.
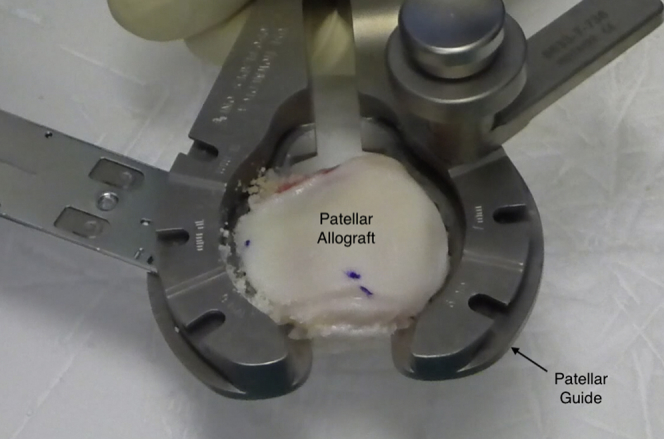
Right patellar graft preparation. The articular side of the patella is resected using a standard patellar guide such as that used in total knee arthroplasty.
The articular side of the trochlear graft is outlined with the sterile skin marker. Three 3 Kirschner wires, on each lateral side of the trochlear joint surface oriented to 45° toward the center of the trochlea from anterior to posterior (Fig 2), serve as cutting guides. The articular side of the trochlea is then resected with a saw and chisels. It is also crucial to remove all the unnecessary soft tissue and perform high-pressure pulsatile lavage irrigation with sterile saline solution to decrease graft immunogenicity.
Fig 2.
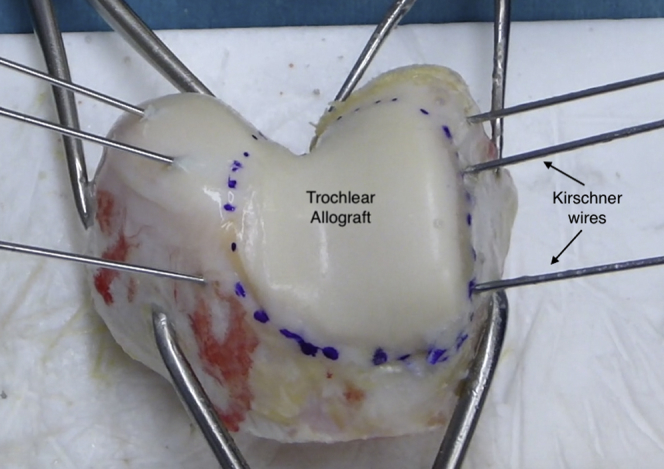
Right trochlear graft preparation. The articular side of the trochlea is outlined with a sterile skin marker. The osteotomy will be performed guided by 3 K-wires placed on each side of the joint surface and oriented to 45° toward the center of the trochlea from anterior to posterior.
Arthroscopic Assessment
The tourniquet is now inflated. An arthroscopic evaluation of all compartments of the knee is first performed to reconfirm that bipolar fresh OCA transplantation of the patella and trochlea is suitable and that there are no excluding criteria that may have been overlooked preoperatively.
Any anatomic deformity or biomechanical alteration of the patellofemoral joint must be corrected to avoid further cartilage degradation of the graft. These corrections may be addressed either in a previous operation or, preferably, concomitantly with the procedure.
Receiving Area Preparation
A longitudinal midline incision is used, and either a standard medial parapatellar approach or a subvastus approach can be performed. Dissection of soft tissues is performed to allow for patellar eversion, with care taken not to excessively remove tissue from the Hoffa fat pad because it is a source of vascularization for the knee extensor apparatus.
The knee is fully extended and the patella is kept everted by twisting it with 2 atraumatic clamps at the level of the insertions of the patellar and quadriceps tendons. Careful measurement of the patellar thickness is performed with a caliper. Superior-inferior and medial-lateral measures are also assessed to check that they match the donor's size and to avoid any instability or pain due to mismatching or overhang. Circumferential denervation on the medial, lateral, and proximal aspects of the patella is performed to decrease postoperative anterior knee pain.
At this point, using the same cutting guide and saws used to prepare the patellar allograft, the articular side of the patella is resected (Fig 3). It is crucial that the resected thickness is never less than that of the allograft; otherwise, an overstuffed patella might increase the patellofemoral joint pressure.
Fig 3.
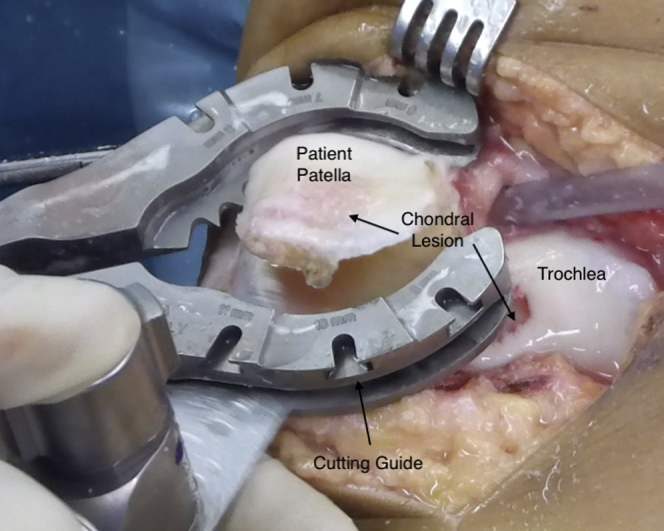
Medial view of right knee. The patellar osteotomy is performed using a standard cutting guide. Care is also taken to position the cutting guide to eliminate only 6 to 8 mm of the subchondral bone tissue.
Subsequently, the knee is flexed at 45° and the patella is everted, exposing the trochlea. The shape and size of the allograft's trochlea are reproduced in the patient's trochlea using a sterile skin marker (Fig 4). Preparation of the trochlea's resection is performed by the same technique used to prepare the trochlear allograft. Meticulous trimming and smoothing of both the recipient and donor subchondral bones must be performed to ensure a perfect match and full bone contact.
Fig 4.

Frontal view of right knee. The knee is flexed at 45° and the patella is everted, exposing the trochlea. The shape and size of the allograft's trochlea are reproduced in the patient's trochlea using a sterile skin marker.
Graft Placement
When the best position for the trochlear transplant is determined, fixation is accomplished with two 3.5-mm headless titanium compression screws with various thread pitches (Acutrak Standard; Acumed, Hillsboro, OR) to provide strong fixation. This is complemented with three or four 2-mm-diameter by 20- to 25-mm-long absorbable pins (SmartNail; ConMed, Largo, FL) (Fig 5).
Fig 5.
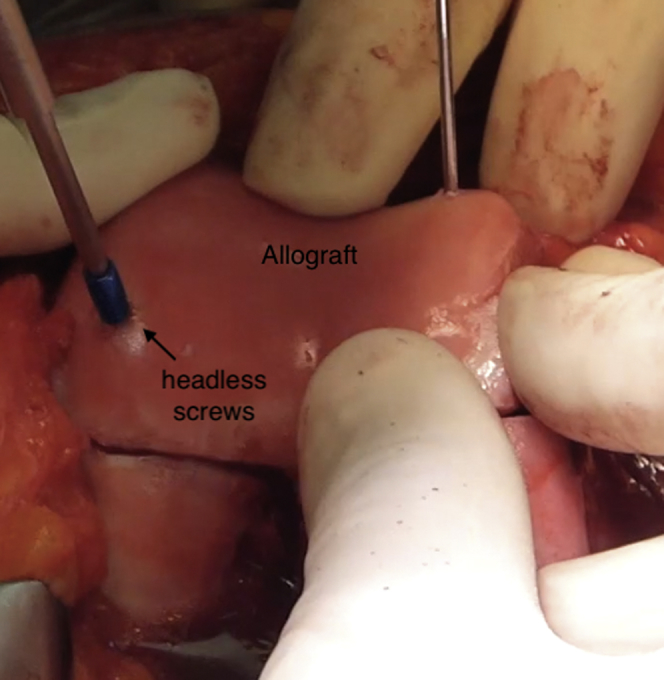
Frontal view of right knee. Trochlear allograft fixation is accomplished with two 3.5-mm headless titanium compression screws.
Once the best position for the patellar transplant is visually determined, temporarily fixing it with two 1.8-mm K-wires positioned on the dorsal aspect of the patella (anterior to posterior) is recommended while care is taken to avoid cartilage tissue piercing (Fig 6). After that, final fixation of the graft can be carried out. Absorbable pins, 1.5 mm in diameter by 16 to 20 mm long (SmartNail), positioned in an anterograde manner through the cartilage surface (from posterior to anterior), are recommended to minimize chondral damage and allow for postoperative magnetic resonance imaging evaluation. We suggest using 4 absorbable pins at the level of each corner of the patella to give proper stability without damaging the cartilage surface implicated in gliding over the trochlear groove. Fixation from the dorsal aspect of the patella with small metal screws can also be performed as an alternative. Once the patellar graft is fixed, the K-wires are removed (Fig 7).
Fig 6.
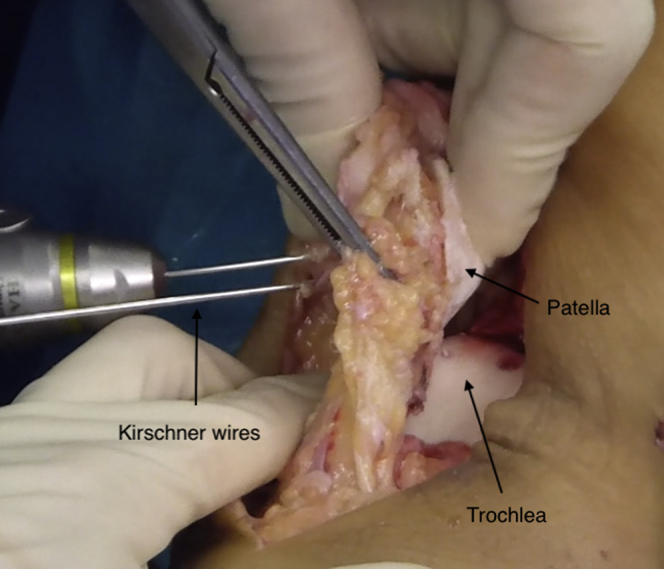
Temporary fixation of the patellar graft is accomplished with two 1.8-mm K-wires positioned on the dorsal aspect of the patella (anterior to posterior) while care is taken to avoid cartilage tissue piercing.
Fig 7.
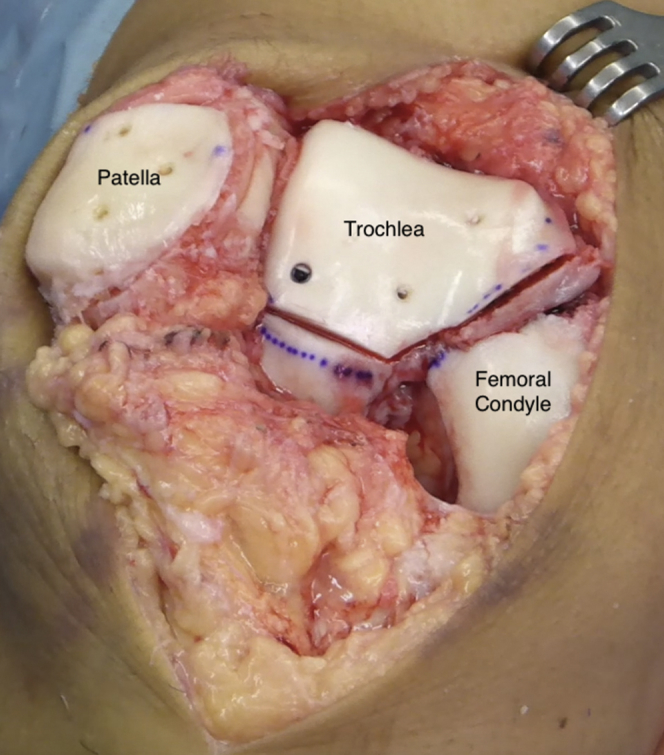
Frontal view of right knee. Final disposition of patellar and trochlear grafts.
Intra-articular drainage is left in place, and the incision is closed. Special attention should be paid to respecting the synovium and the capsular layer. If needed, the vastus medialis muscle may be partially advanced over the patella to decrease lateral facet pressure. The knee is finally immobilized in a brace locked in full extension.
A step-by-step description of the surgical technique is summarized in Table 1. Table 2 provides pearls and pitfalls of performing this procedure. Video 1 shows the whole technique in detail.
Table 1.
Step-by Step Fresh Osteochondral Allograft Resurfacing of Patella and Trochlea
| Step | Description |
|---|---|
| 1 | The patient is positioned supine on the operating table, with 45° of knee flexion, using a distal foot support and a lateral support for the thigh. An arthroscopic evaluation of all compartments of the knee is first performed to reconfirm that bipolar fresh osteochondral allograft transplantation of the patella and trochlea is suitable. |
| 2 | A standard medial parapatellar approach or a subvastus approach can be used. |
| 3 | The knee is extended and the patella is kept everted by twisting it with 2 atraumatic clamps at the level of the insertions of the quadriceps and patellar tendons. |
| 4 | Careful measurement of the patellar thickness is performed with a caliper to maintain the offset of the patellofemoral joint. |
| 5 | An osteotomy is performed using a standard patellar guide such as that used in total knee arthroplasty. Care is also taken to position the cutting guide to eliminate only 6 to 8 mm of the subchondral bone tissue. |
| 6 | Circumferential denervation of the patella is finally performed to decrease postoperative anterior knee pain. |
| 7 | The graft is cut to a thickness that is the same as or slightly less than that of the tissue removed from the patient's patella to maintain the patellofemoral offset. With a sterile skin marker, short lines are drawn on the proximal and lateral part of the graft to aid in its proper placement. |
| 8 | Once the most appropriate position for the transplant is visually determined, fixation is achieved with two to three 1.8-mm K-wires positioned on the dorsal aspect of the patella (anterior to posterior) while care is taken to avoid cartilage tissue piercing |
| 9 | The use of 4 absorbable pins at the level of each corner of the patella is suggested to give absolute stability without damaging the cartilage surface implicated in gliding over the trochlear groove. Once the graft is fixed, the K-wires are removed and patellofemoral tracking, as well as implant stability, is tested again. |
| 10 | The articular side of the trochlear allograft is outlined with a sterile skin marker. The preparation of the trochlea's allograft is performed by fixing 3 K-wires on each lateral side of the joint surface oriented 45° toward the center of the trochlea from anterior to posterior. |
| 11 | The articular side of the trochlea is resected with a saw and chisels, following the guide of the K-wires. The preparation of the trochlea's receiving area resection is performed using the same technique used to prepare the trochlear allograft. |
| 12 | The approach is closed with attention paid to respecting the synovium and the capsular layer. |
Table 2.
Pearls, Pitfalls, and Risks
| Pearls |
| Obtaining osteochondral tissue from donors aged >45 yr is not recommended. |
| Allograft sizing is performed in accordance with a preoperative CT scan (both of the patient and of the graft) and anthropometric agreement between the donor and recipient. |
| The tourniquet should only be inflated once the graft preparation has been finished. |
| Care should be taken to position the cutting guide to eliminate only 6-8 mm of the subchondral bone tissue. This step is of utmost importance because the thicker the bone tissue, the greater the possibility of an immunoreaction. |
| Correction of any anatomic deformity or biomechanical alteration of the patellofemoral joint is mandatory to avoid further cartilage degradation of the graft. Any preoperative TT-TG distance can be used as a guide, but one should consider substantial changes after the new trochlea has been transplanted. |
| To help diminish some degree of immunoreaction, the surgeon should carefully remove remnant soft tissue, use a high-pressure pulsatile irrigation system to eliminate any trace of blood, and transplant a graft with the least amount of bone possible. |
| Suction drainage is used to minimize the risk of hematoma. |
| Circumferential denervation of the patella further helps in preventing residual anterior knee pain. |
| Pitfalls and risks |
| There are no cutting guides for the trochlea. This is a demanding technique. |
| There is a risk of distal osteochondral fracture while preparing the trochlear recipient area near the intercondylar notch. |
| Perfect matching can take several steps by trimming and smoothing the subchondral bone. |
| Should the graft, by any chance, fall on the floor, we recommend a new washing process with the high-pressure pulsatile irrigation system for no less than 20 min, with subsequent immersion in vancomycin solution, 1 g/100 mL, for 10 min. |
| Athletic activity should be limited to light sports. Pivoting and strenuous activities are not recommended. |
| Potentially, the procedure can lead to some degree of immunoreaction. |
CT, computed tomography; TT-TG, tibial tuberosity–trochlear groove.
Rehabilitation Protocol
Controlled continuous passive motion is started within the first hours after the intervention and is used for more than 6 hours per day during the first 6 weeks. Full range of motion is allowed from the beginning. Isometric strengthening of the quadriceps and hamstring muscles is also recommended starting from the first days after graft implantation. Full weight bearing is allowed but only with a brace locked in full extension during the first 6 weeks. At that point, rehabilitation focuses on restoring full range of motion and strengthening.
Pivoting and strenuous activities are not recommended. It is imperative that the patient be made aware of such limitations before OCA transplantation of the patella and trochlea is considered.
Discussion
The treatment of large, symptomatic chondral defects of dysplastic patellofemoral joints in young patients with full bipolar fresh OCA transplantation of the patella and trochlea provides a high number of vital chondrocytes within the extracellular matrix of the cartilage while restoring the normal patellofemoral shape and biomechanics. It permits achieving symptomatic and functional improvement with the possibility of delaying or eliminating the need for prosthetic surgery.
Preventing the migration of multipotent cells from the subchondral bone of the host is achieved by the lack of chondral tissue vascularization and the hard consistency of its matrix. Thus, a graft with the best chondrocyte viability is the best option.16 Fresh-frozen and cryopreservation storage techniques have the advantage of being widely available and allowing for the preservation of the graft for several months. However, it has been shown that these techniques affect the biomechanics and viability of the chondral layer of the graft in an irreversible manner.17
Conversely, fresh OCAs are biomechanically and histologically comparable with autografts and retain viable chondrocytes.18 Thus, fresh allografts are preferred. The downside of fresh allografts is the logistic limitation because the storage time is limited to a few weeks from harvesting.13, 19
A list including the main advantages and disadvantages of the described technique is shown in Table 3. Some tricks and pearls that help to diminish the degree of immunoreaction in the transplant are (1) the use of high-pressure pulsatile lavage irrigation with sterile saline solution to help to eliminate any trace of blood, (2) careful removal of remnant soft tissue, and (3) transplanting a graft with a bone layer no thicker than 6 to 10 mm. In addition, osteochondral integration is only achieved owing to the creeping substitution phenomenon in this thin layer of bone. The bone will be subjected to a necrosis process with subsequent revascularization during the healing progress.15
Table 3.
Advantages and Limitations
| Advantages |
| The treatment is biological. |
| The procedure is indicated in young subjects with extensive cartilage or osteochondral lesions in the patella and trochlea (kissing lesions) with Dejour type C or D trochlear dysplasia that cannot be treated with other, less aggressive techniques. |
| Good results are achieved when performed with a surgical technique that follows a few standard steps to maintain the long-term viability of the graft. |
| Fresh osteochondral allografts are biomechanically and histologically comparable with autografts and retain viable chondrocytes. |
| Implantation of a patellofemoral arthroplasty is prevented. |
| Limitations |
| The use of fresh allografts carries considerable logistic limitations, and this material is not easily available worldwide. |
| The main exclusion criteria are advanced osteoarthritis of other compartments of the knee and general conditions such as infections, tumors, locally aggressive rheumatic disease, diabetes, and vasculitis. |
| Relative contraindications are BMI >30 and age >50 yr. Smoking must be stopped 30 d before surgery and abstained from for at least 6 mo after the operation. |
| Only patients who have severe chronic pain that limits their daily activities and see no improvement with rehabilitative treatment are candidates for this treatment. |
| The technique is not intended for patients seeking to return to demanding pivoting activities. |
BMI, body mass index.
Correction of any anatomic deformity or biomechanical alteration of the patellofemoral joint is mandatory to avoid further cartilage degradation of the graft. Special attention must be paid to the tibial tuberosity–trochlear groove distance because the transplanted trochlea will modify the preoperative value. Subsequently, a proper intraoperative assessment of patellofemoral tracking must be performed. Any obvious maltracking with a high Q angle can be corrected with tibial tuberosity medialization.
Studies on bipolar OCA transplantation of the patella and trochlea are few and have been limited to fewer than 20 patients. In addition, most of them have reported surgical techniques using bone plugs instead of the described resurfacing technique. Risk of failure is increased because of the size of the joint. However, patients with surviving allografts showed significant improvements in functional outcomes, pain relief, and range of motion.20, 21, 22, 23, 24, 25
In conclusion, the current technique is a valid option for severe anterior knee pain due to large cartilage defects in both the patella and the dysplastic trochlea in young patients. Although the technique is relatively demanding, it can be performed perfectly well by following the described steps. This would allow for delaying or even avoiding the need for an onlay-design patellofemoral arthroplasty.
Footnotes
The authors report the following potential conflicts of interest or sources of funding: P.E.G. is a consultant for ConMed. J.C.M. receives grants/grants pending from Spanish Ministerio de Economia,Industria y Competitividad (National Programme for Research Aimed at the Challenges of Society), and payments for lectures from Smith & Nephew. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
Two cases comprising 24- and 27-year-old female patients who have severe knee pain due to patellofemoral osteoarthritis owing to a high degree of dysplasia that can easily be seen on magnetic resonance imaging, a computed tomography scan, and an arthroscopic view. A new chondral layer and a normal shape of the patellofemoral joint were needed, so we decided to perform a fresh osteochondral patellofemoral resurfacing technique. The fresh osteochondral allograft is delivered, and preparation then starts. The patella is prepared similarly to a patellar arthroplasty in a total knee replacement. Once the resection has been performed, it is important that as much soft tissue is resected as possible to decrease a potential immune response. Then, we keep it in a container for posterior high-pressure pulsatile lavage. On the other hand, the trochlear resection is a much more demanding part of the surgical procedure because there is no cutting guide developed for this purpose. First, the periphery of the desired cut is drawn with a pen; then, 3 or 4 K-wires are placed on both sides at 40° of angulation to be used as cutting guides. A tip to avoid excessive cutting is to measure the required depth and then draw a limit line on the saw. We finish the cut using a chisel to avoid any fracture. Then, we proceed with the host site preparation, using an anterior approach and a medial arthrotomy. We can observe, in the trochlea, grade C dysplasia. In the patient's patella, the surgeon performs the same resection as in the allograft using the same cutting guide and saw. The surgeon presents the allograft of the trochlea and tries to reproduce the cut in the patient joint by drawing the same shape with a pen. As in the allograft, 3 K-wires are placed on both sides of the trochlea and the cut is performed with a saw and chisel. Meticulous trimming and smoothing of both the recipient and donor subchondral bones must be performed to ensure a perfect match and full bone contact. When the best position for the trochlear transplant is determined, fixation is accomplished with two 3.5-mm headless titanium compression screws with various thread pitches. It is complemented with three or four 2-mm-diameter by 20- to 25-mm-long absorbable pins. Once the best position for the patellar transplant is visually determined, it is temporarily fixed with two 1.8-mm K-wires positioned on the dorsal aspect of the patella. After that, final fixation of the graft can be carried out. Absorbable pins, 1.5 mm in diameter by 16 to 20 mm long, are positioned in an anterograde manner through the cartilage surface. Once the patellar graft is fixed, the K-wires are removed. Intra-articular drainage is left in place, and the incision is closed. If needed, the vastus medialis muscle may be partially advanced over the patella to decrease lateral facet pressure. The knee is finally immobilized in a brace locked in full extension.
References
- 1.Timothy L., Sébastien L., Elvire S., Philippe N. Chondral injury in patellofemoral instability. Cartilage. 2014;5:136–144. doi: 10.1177/1947603514530142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mestriner A.B., Ackermann J., Gomoll A.H. Patellofemoral cartilage repair. Curr Rev Musculoskelet Med. 2018;11:188–200. doi: 10.1007/s12178-018-9474-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Draper C., Bezier T., Gold G., Fredericson M., Fiene A., Beaupre A. Is cartilage thickness different in young subjects with and without patellofemoral pain? Osteoarthritis Cartilage. 2006;14:931–937. doi: 10.1016/j.joca.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Mason J.J., Leszko F., Johnson T., Komistek R.D. Patellofemoral joint forces. J Biomech. 2008;41:2337–2348. doi: 10.1016/j.jbiomech.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 5.Brophy R.H., Wojahn R.D., Lamplot J.D. Cartilage restoration techniques for the patellofemoral joint. J Am Acad Orthop Surg. 2017;25:321–329. doi: 10.5435/JAAOS-D-15-00447. [DOI] [PubMed] [Google Scholar]
- 6.Hangody L., Vásárhelyi G., Hangody L.R. Autologous osteochondral grafting. Technique and long-term results. Injury. 2008;39:S32–S39. doi: 10.1016/j.injury.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 7.Gudas R., Gudaite A., Pocius A. Ten-year follow-up of a prospective, randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint of athletes. Am J Sports Med. 2012;40:2499–2508. doi: 10.1177/0363546512458763. [DOI] [PubMed] [Google Scholar]
- 8.Filardo G., Kon E., Andriolo L., Di Martino A., Zaffagnini S., Marcacci M. Treatment of “patellofemoral” cartilage lesions with matrix-assisted autologous chondrocyte transplantation: A comparison of patellar and trochlear lesions. Am J Sports Med. 2014;42:626–634. doi: 10.1177/0363546513510884. [DOI] [PubMed] [Google Scholar]
- 9.Lustig S. Patellofemoral arthroplasty. Orthop Traumatol Surg Res. 2014;100:35–43. doi: 10.1016/j.otsr.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 10.Zouzias I.C., Bugbee W.D. Osteochondral allograft transplantation in the knee. Sports Med Arthrosc Rev. 2016;24:79–84. doi: 10.1097/JSA.0000000000000109. [DOI] [PubMed] [Google Scholar]
- 11.Bugbee W.D., Pallante-Kichura A.L., Gortz S., Amiel D., Sah R. Osteochondral allograft transplantation in cartilage repair: Graft storage paradigm, translational models, and clinical applications. J Orthop Res. 2016;34:31–38. doi: 10.1002/jor.22998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gross A.E., Kim W., Las Heras F., Backstein D., Safir O., Pritzker K.P. Fresh osteochondral allografts for posttraumatic knee defects: Long-term followup. Clin Orthop Relat Res. 2008;466:1863–1870. doi: 10.1007/s11999-008-0282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmidt S., Schulte A., Schwarz S. Fresh osteochondral allografts-procurement and tissue donation in Europe. Injury. 2017;48:1296–1301. doi: 10.1016/j.injury.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Zou S., Dodd R.Y., Stramer S.L., Strong D.M., Tissue Safety Study Group Probability of viremia with HBV, HCV, HIV, and HTLV among tissue donors in the United States. N Engl J Med. 2004;351:751–759. doi: 10.1056/NEJMoa032510. [DOI] [PubMed] [Google Scholar]
- 15.Riberts T.T., Rosenbaum A.J. Bone grafts, bone substitutes and orthobiologics. The bridge between basic science and clinical advancements in fracture healing. Organogenesis. 2012;8:114–124. doi: 10.4161/org.23306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Caro F., Bisicchia S., Amendola A., Ding L. Large fresh osteochondral allografts of the knee: A systematic clinical and basic science review of the literature. Arthroscopy. 2015;31:757–767. doi: 10.1016/j.arthro.2014.11.025. [DOI] [PubMed] [Google Scholar]
- 17.Pallante-Kichura A.L., Chen A.C., Temple-Wong M.M., Bugbee W.D., Sah R.L. In vivo efficacy of fresh versus frozen osteochondral allografts in the goat at 6 months is associated with PRG-4 secretion. J Orthop Res. 2013;31:880–886. doi: 10.1002/jor.22319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCarty E.C., Fader R.R., Mitchell J.J., Glenn R.E., Jr., Potter H.G., Spindler K.P. Fresh osteochondral allograft versus autograft: Twelve-month results in isolated canine knee defects. Am J Sports Med. 2016;44:2354–2365. doi: 10.1177/0363546516648700. [DOI] [PubMed] [Google Scholar]
- 19.Williams S.K., Amiel D., Ball S.T. Prolonged storage effects on the articular cartilage of fresh human osteochondral allograft. J Bone Joint Surg Am. 2003;85:2111–2120. doi: 10.2106/00004623-200311000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Jamali A.A., Emmerson B.C., Chung C., Convery F.R., Bugbee W.D. Fresh osteochondral allografts: Results in the patellofemoral joint. Clin Orthop Relat Res. 2005;437:176–185. [PubMed] [Google Scholar]
- 21.Torga Spak R., Teitge R.A. Fresh osteochondral allografts for patellofemoral arthritis: Long-term followup. Clin Orthop Relat Res. 2006;444:193–200. doi: 10.1097/01.blo.0000201152.98830.ed. [DOI] [PubMed] [Google Scholar]
- 22.Giannini S., Buda R., Ruffilli A. Failures in bipolar fresh osteochondral allograft for the treatment of end-stage knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2015;23:2081–2089. doi: 10.1007/s00167-014-2961-1. [DOI] [PubMed] [Google Scholar]
- 23.Meric G., Gracitelli G.C., Gortz S., De Young A.J., Bugbee W.D. Fresh osteochondral allograft transplantation for bipolar reciprocal osteochondral lesions of the knee. Am J Sports Med. 2015;43:709–714. doi: 10.1177/0363546514562549. [DOI] [PubMed] [Google Scholar]
- 24.Mirzayan R., Charles M.D., Batech M., Suh B.D., DeWitt D. Bipolar osteochondral allograft transplantation of the patella and trochlea. Cartilage. 2018 doi: 10.1177/1947603518796124. 1947603518796124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Assenmacher A.T., Pareek A., Reardon P.J., Macalena J.A., Stuart M.J., Krych A.J. Long-term outcomes after osteochondral allograft: A systematic review at long-term follow-up of 12.3 years. Arthroscopy. 2016;32:2160–2168. doi: 10.1016/j.arthro.2016.04.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Two cases comprising 24- and 27-year-old female patients who have severe knee pain due to patellofemoral osteoarthritis owing to a high degree of dysplasia that can easily be seen on magnetic resonance imaging, a computed tomography scan, and an arthroscopic view. A new chondral layer and a normal shape of the patellofemoral joint were needed, so we decided to perform a fresh osteochondral patellofemoral resurfacing technique. The fresh osteochondral allograft is delivered, and preparation then starts. The patella is prepared similarly to a patellar arthroplasty in a total knee replacement. Once the resection has been performed, it is important that as much soft tissue is resected as possible to decrease a potential immune response. Then, we keep it in a container for posterior high-pressure pulsatile lavage. On the other hand, the trochlear resection is a much more demanding part of the surgical procedure because there is no cutting guide developed for this purpose. First, the periphery of the desired cut is drawn with a pen; then, 3 or 4 K-wires are placed on both sides at 40° of angulation to be used as cutting guides. A tip to avoid excessive cutting is to measure the required depth and then draw a limit line on the saw. We finish the cut using a chisel to avoid any fracture. Then, we proceed with the host site preparation, using an anterior approach and a medial arthrotomy. We can observe, in the trochlea, grade C dysplasia. In the patient's patella, the surgeon performs the same resection as in the allograft using the same cutting guide and saw. The surgeon presents the allograft of the trochlea and tries to reproduce the cut in the patient joint by drawing the same shape with a pen. As in the allograft, 3 K-wires are placed on both sides of the trochlea and the cut is performed with a saw and chisel. Meticulous trimming and smoothing of both the recipient and donor subchondral bones must be performed to ensure a perfect match and full bone contact. When the best position for the trochlear transplant is determined, fixation is accomplished with two 3.5-mm headless titanium compression screws with various thread pitches. It is complemented with three or four 2-mm-diameter by 20- to 25-mm-long absorbable pins. Once the best position for the patellar transplant is visually determined, it is temporarily fixed with two 1.8-mm K-wires positioned on the dorsal aspect of the patella. After that, final fixation of the graft can be carried out. Absorbable pins, 1.5 mm in diameter by 16 to 20 mm long, are positioned in an anterograde manner through the cartilage surface. Once the patellar graft is fixed, the K-wires are removed. Intra-articular drainage is left in place, and the incision is closed. If needed, the vastus medialis muscle may be partially advanced over the patella to decrease lateral facet pressure. The knee is finally immobilized in a brace locked in full extension.


