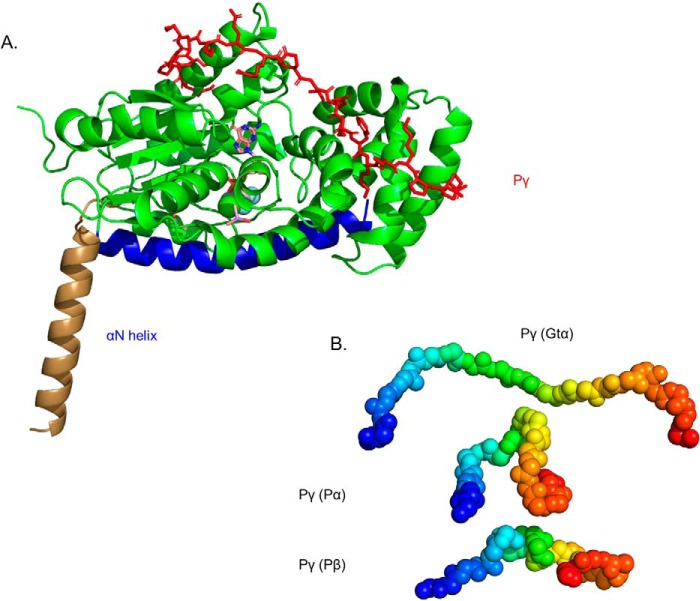
Figure 2.
Structural model of Gtα–GDP–AlF4− and its interaction with Pγ in solution. A, the structural model of Gtα was determined using the 1TAD crystal structure as the template (36) and refined with spatial restraints imposed from cross-linking results in the absence or presence of Pγ (Table 2). Structural elements that were unchanged in the cross-link–refined model are represented in green, with the conformational change of the αN helix shown in brown (for the crystal structure) and blue (for the cross-link modified solution structure). Also shown is the docked structure of Pγ (red) with Gtα–GDP–AlF4− based on the observed cross-linking results when Gtα associated with lipobeads was incubated with a 2-fold molar excess of Pγ. Note that no significant changes in Gtα conformation were observed upon Pγ binding. B, a comparison of the conformation of the central region of Pγ (residues 24–44, depicted as a gradient from blue to red spheres) when bound to Gtα or to the PDE6 catalytic subunits.