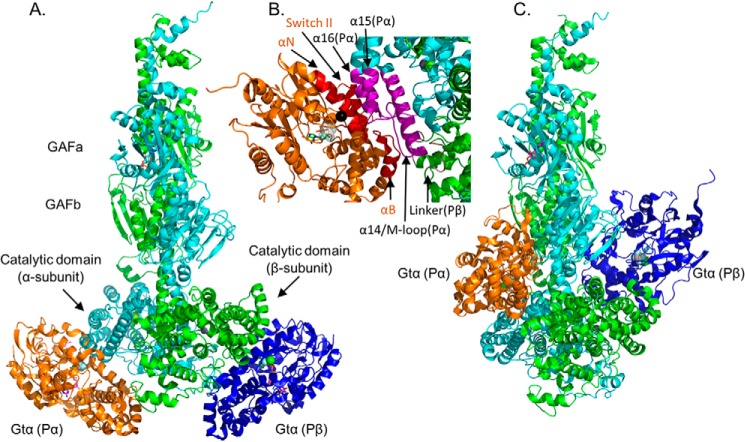
Figure 3.
Model of Gtα–GDP–AlF4− docked to the Pαβ catalytic dimer. PDE6 holoenzyme and Gtα–GDP–AlF4− bound to lipobeads (see “Experimental procedures”) were exposed to chemical cross-linkers, and the identified cross-linked peptides between Gtα and PDE6 subunits (Table 3) were then used as spatial restraints for integrative structural modeling. Two predominant clusters of models of the Gtα–Pαβ complex were generated, one with Gtα docked to the two catalytic domains (with distance violations for Gtα24-Pα330/Pβ328, Gtα9-Pα442, and Gtα9-Pβ440) and the other with Gtα docked to the GAFb domains (with distance violations for Gtα10-Pα854, Gtα17-Pα551, and Gtα128-Pα807/Pβ817). Because of insufficient cross-links for Pγ in the activated complex, the inhibitory subunit is not shown. A, structural model of association of Gtα–GDP–AlF4− to the α-subunit (Gtα(Pα), orange) and to the β-subunit (Gtα(Pβ), blue) catalytic domains. B, detailed view of the Gtα GTPase subdomain interface with the α-subunit catalytic domain, with the interaction surface of Gtα colored red and the α- and β-subunit interacting residues colored magenta and brown, respectively. The black sphere indicates Gtα Gln200. C, alternate docking of Gtα to the GAFb domains of the Pαβ catalytic dimer (with the same orientation as in A).