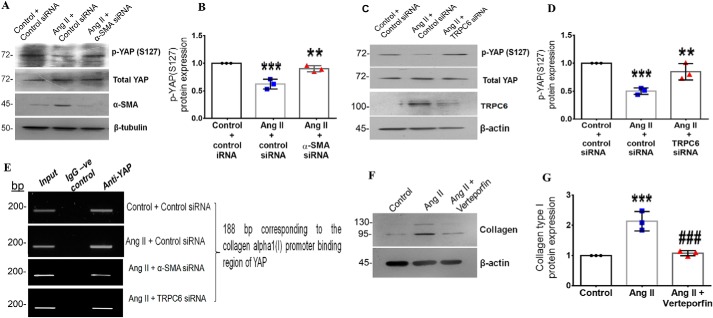
Figure 8.
α-SMA–dependent TRPC6 regulates collagen type I expression via YAP activation in Ang II–treated cardiac fibroblasts. Cardiac fibroblasts were transiently transfected with α-SMA siRNA, TRPC6 siRNA, or scrambled siRNA (control). A and B, following exposure of the α-SMA siRNA-transfected cells to Ang II for 6 h, phosphorylation of YAP at Ser-127 was examined by Western blot analysis and normalized to total YAP. ***, p < 0.001 versus control; **, p < 0.01 versus Ang II. C and D, following exposure of the TRPC6 siRNA–transfected cells to Ang II for 6 h, phosphorylation of YAP at Ser-127 was examined by Western blot analysis and normalized to total YAP. ***, p < 0.001 versus control; **, p < 0.01 versus Ang II. E, cardiac fibroblasts were transiently transfected with α-SMA siRNA, TRPC6 siRNA, or scrambled siRNA (control). Following Ang II treatment for 6 h, the ChIP assay was performed as described under “Experimental procedures.” DNA binding of YAP to the collagen α1(I) gene promoter was confirmed using anti-YAP antibody. A nonspecific anti-rabbit IgG was used as negative control. A representative image showing the PCR amplification product is shown. F and G, subconfluent quiescent cultures of cardiac fibroblasts were pretreated with the YAP inhibitor (verteporfin) for 1 h and, subsequently, with Ang II. Protein was isolated at 12 h post-Ang II treatment and subjected to Western blot analysis for detection of collagen α1(I), with β-actin as loading control. ***, p < 0.001 versus control; ###, p < 0.001 versus Ang II. Data are representative of three independent experiments (n = 3). Error bars, S.D.