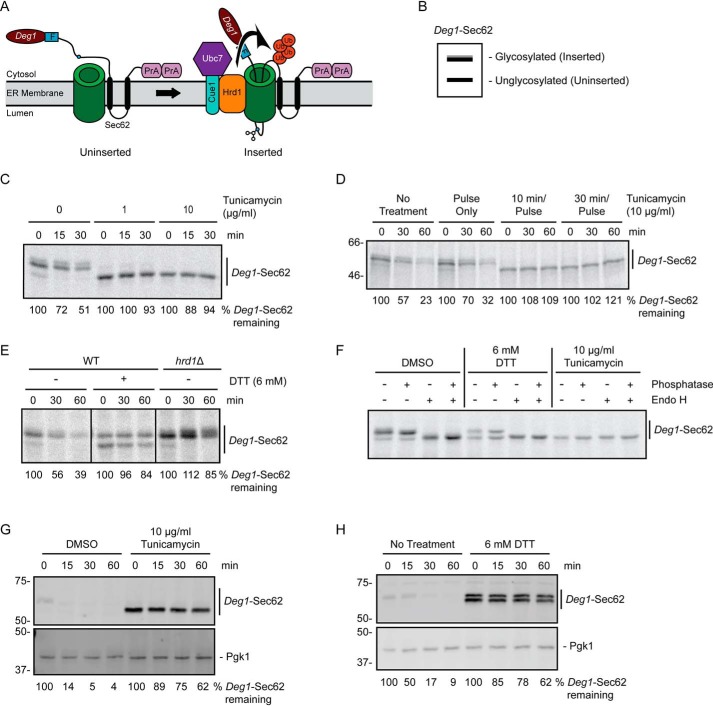
Figure 1.
ER stress impairs degradation of a Hrd1 ERAD-T substrate. A, schematic depiction of Deg1-Sec62 prior to (uninserted) and following (inserted) aberrant translocon engagement. Deg1-Sec62 consists of Deg1 (the N-terminal 67 amino acids from the yeast transcriptional repressor MATα2), a FLAG (F) epitope, the two-transmembrane protein Sec62, and two copies of the S. aureus protein A (PrA). Following translocon engagement, Deg1-Sec62 undergoes extensive PTM, including N-linked glycosylation, and is polyubiquitylated by Hrd1. The primary glycosylated asparagine residue is indicated as a blue circle. Ub, ubiquitin. B, virtual SDS-PAGE illustrates differential migration of Deg1-Sec62 based on glycosylation status. C, pulse-chase analysis of WT yeast expressing Deg1-Sec62 cultured in the presence of DMSO or tunicamycin at the indicated concentrations for 30 min. DMSO and tunicamycin were maintained at the same concentrations throughout pulse labeling. D, pulse-chase analysis of WT yeast expressing Deg1-Sec62 cultured in the presence of 10 μg/ml tunicamycin for the indicated times (or DMSO for 30 min). Tunicamycin and DMSO were maintained at the same concentrations throughout pulse labeling. E, pulse-chase analysis of WT or hrd1Δ yeast expressing Deg1-Sec62 cultured in the presence of 6 mm DTT (or no treatment) for 30 min. DTT was maintained at the same concentrations throughout pulse labeling. F, doa10Δ hrd1Δ yeast cells expressing Deg1-Sec62 were cultured in the presence 6 mm DTT, 10 μg/ml tunicamycin, or DMSO control for 30 min. DTT, tunicamycin, and DMSO were maintained at the indicated concentrations throughout pulse labeling. Immunoprecipitated Deg1-Sec62 was incubated in the presence or absence of Endo H and calf intestinal phosphatase as indicated. G and H, cycloheximide chase analysis of WT yeast expressing Deg1-Sec62 cultured in the presence of 10 μg/ml tunicamycin or DMSO (G) or 6 mm DTT or no treatment (H) for 1 h. Tunicamycin, DTT, or DMSO were maintained at the same concentration during incubation with cycloheximide. Deg1-Sec62 was detected with AlexaFluor-680–conjugated rabbit anti-mouse antibodies. Pgk1 served as a loading control. Where indicated, the percentage of Deg1-Sec62 remaining at each time point is presented below the image. For cycloheximide chase experiments, Deg1-Sec62 signal intensity was normalized to Pgk1. The experiment depicted in C was performed three times with tunicamycin at 0 and 10 μg/ml, and one time with tunicamycin at 1 μg/ml. Experiments depicted in D and F were performed one time. Experiments depicted in E, G, and H were performed three times.