Figure 4.
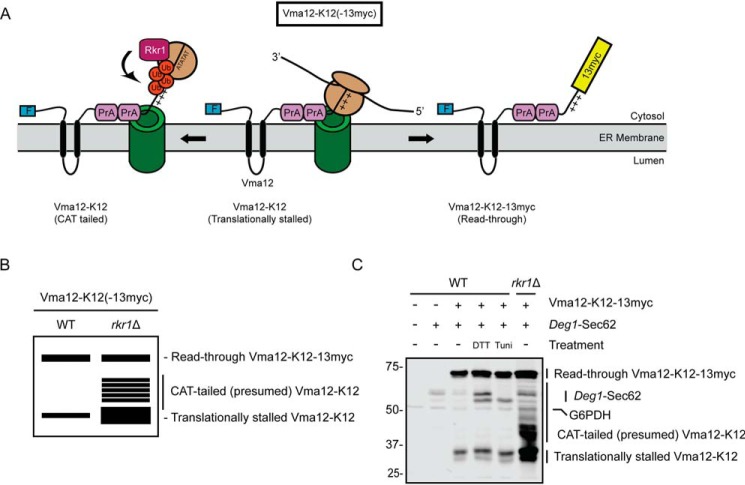
ER stress does not alter abundance of an Rkr1 ERAD-RA substrate. A, schematic of Vma12-K12-13myc, which consists of a FLAG epitope tag (F), the two-transmembrane protein Vma12, two copies of the S. aureus protein A (PrA), 12 lysine (K12) residues (depicted as sequential “+” symbols), and a 13myc epitope tag. After insertion of the two transmembrane segments of Vma12, K12 triggers translational stalling (middle). This is resolved by predicted C-terminal addition of alanine and threonine (AT) residues (CAT tailing), Rkr1-mediated ubiquitylation, and degradation (left) or release from stalling and translation of 13myc (right). Ub, ubiquitin. B, virtual SDS-PAGE illustrates differential migration of translationally stalled Vma12-K12, CAT-tailed Vma12-K12, and read-through product Vma12-K12-13myc in WT and rkr1Δ yeast lysates. C, yeast of the indicated genotypes harboring plasmids encoding Vma12-K12-13myc and Deg1-Sec62 (as a control for ER stress induction) or empty vectors were cultured in the presence of 6 mm DTT, 10 μg/ml tunicamycin, or DMSO (−) for 1 h prior to lysis and separation by SDS-PAGE. Vma12-K12-13myc was detected using anti-Myc antibodies, which bind to both 13myc and protein A. Deg1-Sec62 was detected with AlexaFluor-680–conjugated rabbit anti-mouse antibodies. G6PDH served as a loading control. Expression of Vma12-K12(-13myc) in rkr1Δ cells may increase Deg1-Sec62 levels, suggesting stabilized ERAD-RA substrates may cross-inhibit ERAD-T. The experiment depicted was performed four times.