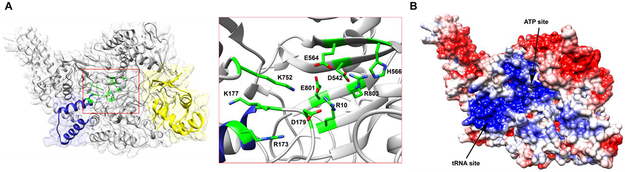
Figure 4 ∣. Phyre2 homology model of TglB.
(A) The overall predicted structure of TglB is shown in grey. The Arg/Lys-rich region putatively involved in tRNA binding is colored in navy. The RRE motif engaged in precursor peptide recognition is colored in yellow. Residues identified to have diminished cysteinylation activity in TglB are clustered in the predicted structure as shown in the insert. (B) Calculated electrostatic potential mapped onto the TglB surface showing the basic patch (blue) that probably engages in ATP and/or Cys-tRNACys binding. The sites of putative ATP and tRNA binding are based on mutagenesis data in this study.