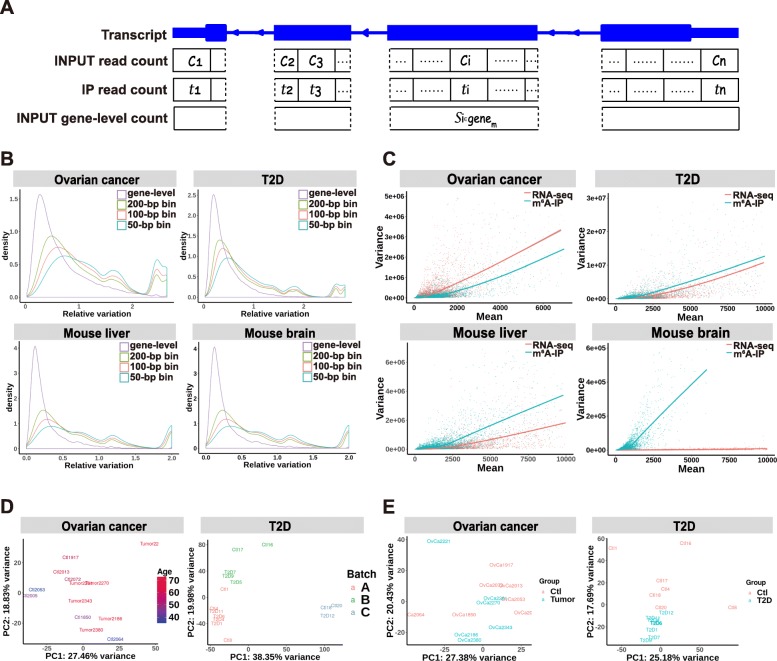
Fig. 1.
Unique features of m6A-seq (MeRIP-seq) data. RADAR divides concatenated exons of a gene into consecutive bins and models the immunoprecipitation (IP)-enriched read counts in such bins. a depicts a pair of read counts in the INPUT and the IP library in the ith bin as ci and ti. In the RADAR workflow, the gene-level read count of the input library substitutes the bin-level read count ci as the representation of the pre-IP RNA levels of the ith bin. b compares the relative variation of gene-level and bin-level (local) read counts of different bin sizes in four m6A-seq datasets, suggesting that unwanted variation can be reduced using gene-level counts as the estimates of pre-IP RNA levels. Panel c compares the cross-sample mean and variance of regular RNA-seq (pre-IP counts) and m6A-seq (post-IP read counts adjusted for pre-IP RNA level variation) data in four m6A-seq datasets. The fitted curvature of m6A-seq can differ from that of RNA-seq, indicating that m6A-seq may have a different mean-variance relationship from RNA-seq. Biological and experimental confounding factors are often encountered in patient samples. d shows the first two principal components (PCs) of m6A enrichment in each dataset, where the samples are colored by covariates that need to be accounted for. m6A enrichment was represented by IP sample read counts adjusted for pre-IP (INPUT) RNA-level variation. e shows the first two PCs after regressing out known covariates—age in the ovarian cancer dataset and batch in the T2D dataset. After regressing out the covariate, samples are separated by disease conditions on the PCA plot