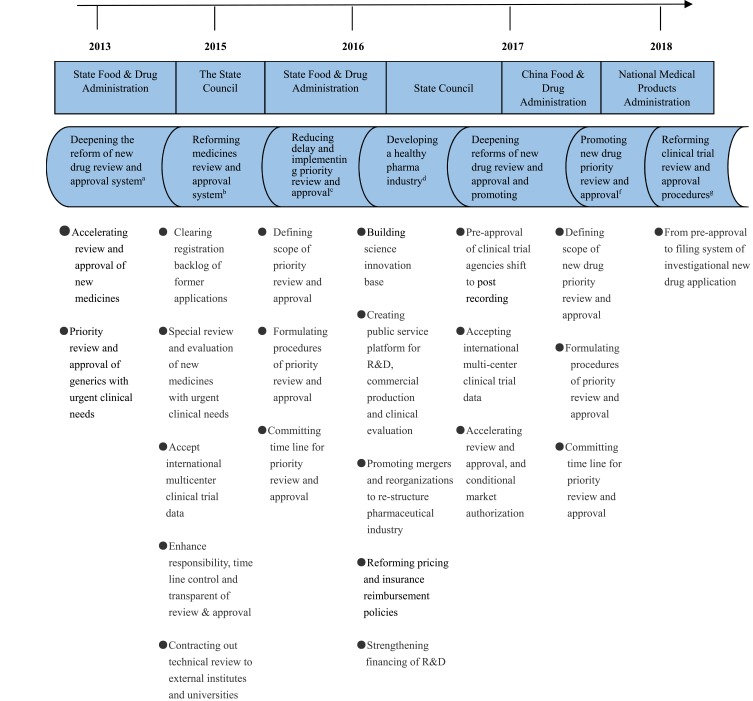
Figure 2.
Milestones and landmark policies to streamline the regulatory process.
Notes: aNational Medical Products Administration. Deepening the1 National Medical Products Administration. Deepening the reform of review and approval system and promoting medicines innovation. Document No. 37 of 2013. http://www.nmpa.gov.cn/WS04/CL2196/323982.html (in Chinese, accessed July 30, 2019). bThe State Council. Opinions of reforming review and approval systems of medicines and medical devices. Document No. 44 of 2015. http://www.gov.cn/zhengce/content/2015-08/18/content_10101.htm (in Chinese, accessed July 30, 2019). cChina Food and Drug Administration. Reducing delay of medicines registration and implementing priority review and approval. Document No. 19 of 2016 (Abolished and replaced by a new document issued in reform of review and approval system and promoting medicines innovation. Document No. 37 of 2013. http://www.nmpa.gov.cn/WS04/CL2196/323982.html (in Chinese, accessed July 30, 2019). dThe State Council. Opinions of reforming review and approval systems of medicines and medical devices. Document No. 44 of 2015. http://www.gov.cn/zhengce/content/2015-08/18/content_10101.htm (in Chinese, accessed July 30, 2019). eChina Food and Drug Administration. Reducing delay of medicines registration and implementing priority review and approval. Document No. 19 of 2016 (Abolished and replaced by a new document issued in 2017). http://samr.cfda.gov.cn/WS01/CL0844/145260.html (in Chinese, accessed July 30, 2019). fThe State Council. Promoting healthy development of the pharmaceutical industry. Document No. 11 of 2016. http://www.gov.cn/zhengce/content/2016-03/11/content_5052267.htm (in Chinese, accessed July 30, 2019). gChinese Communist Party Central Committee and State Council. Opinions to deepening the reform of review and approval system and promoting medicines and medical devices innovation. http://www.gov.cn/zhengce/2017-10/08/content_5230105.htm (in Chinese, accessed July 30, 2019). hNational Medical Products Administration. Promoting new drug priority review and approval. Document No. 126 of 2017. http://www.nmpa.gov.cn/WS04/CL2196/324193.html (in Chinese, accessed July 30, 2019). iNational Medical Products Administration. Reforming clinical trial review and approval procedures. Announcement No.50 of 2018. http://www.nmpa.gov.cn/WS04/CL2111/329716.html (in Chinese, accessed July 30, 2019).