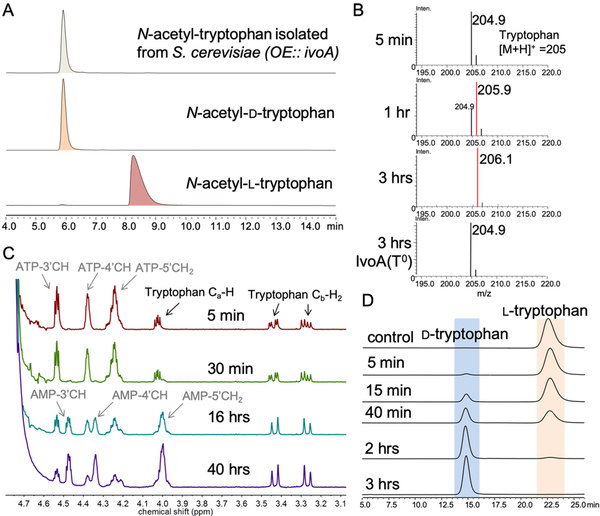
Figure 2.
Characterization of IvoA activity. A) Stereochemistry determination for isolated N-acetyl-d-tryptophan. B) Mass spectrometry shows the mass shift of tryptophan when the assay was performed in D2O. C) 1H-NMR spectra indicate incorporation of deuterium at the α position: 1) the change of splitting pattern of the diastereotopic β proton signal due to smaller coupling constant (3JH-D)); 2) the disappearance of α proton signal. D) Chiral HPLC resolution of tryptophan enantiomers from IvoA reaction demonstrated complete stereoinversion of l-tryptophan to d-tryptophan.