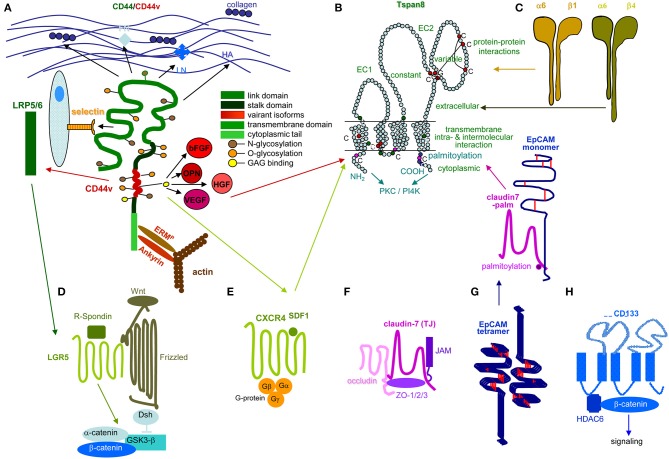
Figure 2.
Prominent PaCIC markers. (A) The lead PaCIC marker is CD44v6, a type I transmembrane protein that contributes to the crosstalk with the ECM via its link domain and the HA binding site. It has binding sites for selectins and LRP5/6. The v6 exon product carries binding sites for several growth factors. The cytoplasmic tail has binding sites for ankyrin and ERM proteins including merlin, which promote cytoskeleton association and downstream signaling. (B) Tspan8 is a tetraspanin with a small and a large extracellular loop, the latter mostly accounts for protein-protein interactions. The four transmembrane regions account for intramolecular and intermolecular interactions. The cytoplasmic tail binds PKC and PI4K. Main activities rely on the association with a large range of proteins. Dominant are integrins, but also CD44v6 and an EpCAM-cldn7 complex. (C) Particularly α6β4 is known as a PaCIC marker. Similar to other integrins, it binds matrix proteins, particularly LN. It is a major component of hemidesmosomes anchoring epithelial cells in the basal membrane. Upon activation, it leaves the desmosome complex and associates preferentially with Tspan8. It differs from other integrins by a long cytoplasmic domain of the β4 chain, which promotes multiple signaling pathways. (D) LGR5 is a seven transmembrane protein located close to frizzled. Upon R-spondin binding, it contributes via Wnt activation to ß-catenin liberation. LGR5 activity is supported by CD44v6-associated LRP5/6. (E) CXCR4 is another seven transmembrane protein. This GPCR becomes activated by SDF1 binding. It predominantly signals via trimeric G-proteins. CD44 crosslinking via HA promotes CXCR4 recruitment and strengthens activation of downstream signaling cascades. Activated CXCR4 also associates with Tspan8 (F) Claudin7 is a 4 transmembrane protein, which can be integrated in TJ, where it associates with other claudins, JAM, and occludin and the cytoplasmic zonula occludens proteins. Cldn7 is also recovered outside of TJ. Upon palmitoylation, it associates via a direct protein-protein interaction in the transmembrane region with monomeric EpCAM. The cldn7-EpCAM complex is recruited into TEM and associates with Tspan8. (G) EpCAM is a type I transmembrane protein of many epithelial cells. It forms tetramers, which promote homophilic binding to EpCAM on neighboring cells. It is engaged in signal transduction, predominantly via the liberated cytoplasmic tail that acts as cotranscription factor. (H) CD133 is a five transmembrane protein located in cholesterol-rich membrane domains. It is associated with HDAC6 that stabilizes a ternary CD133-HDAC6-β-catenin complex and β-catenin target activation, which present one of the signaling cascades initiated via CD133. The seven most prominent PaCIC markers belong to distinct protein families and exert non-related functions. Five of these molecules can become recruited into TEM, where they associate via weak, non-protein-protein interactions with Tspan8. This significantly expands the range of activities of TEM and TEM-derived Exo. Of note, all seven CIC markers contribute via different routes to maintain stem cell features.