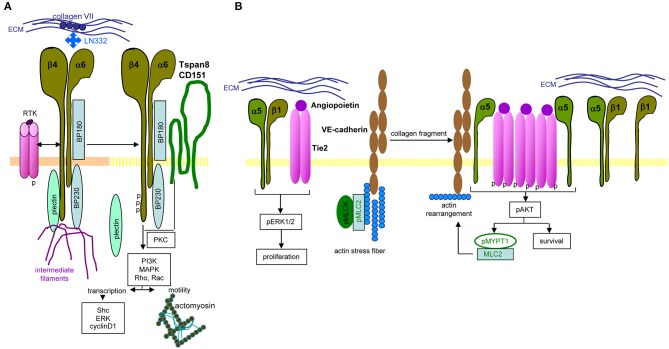
Figure 4.
Distinct integrin signaling in PaCIC. (A) Hemidesomosome-integrated α6β4 is associated with BP160/320 and plectin, the complex being linked to intermediate filament. Upon contact with RTK, the β4 cytoplasmic tail becomes phosphorylated, plectin is released from the complex and phosphorylated β4, supported by Tspan8-associated PKC promotes PI3K, MAPK, Rho, and RAC activation. Besides initiating transcription, the complex assists the association with actomyosin and motility. (B) Instead, when α5β1 associates with angiopoietin-activated Tie2, proliferation is initiated via ERK phosphorylation. In the presence of VE-cadherin, linked to actin stress fibers, pMLCK, and pMLC2 collagen fragments initiate actin rearrangement that promotes dissociation of the α5 from the β1 chain, which enclose phosphorylated Tie2. The phosphorylated Tie2 promotes Akt phosphorylation, which supports MYPT1 phosphorylation and MLC2 association that evoke actin rearrangement. Full name of proteins are listed in Table S1. In brief, only parts of integrin-mediated activities are affected by the association with Tspan8. Notably, the same stimulus distinctly affects integrin activation depending on the α or β chain of the integrin.