Figure 8.
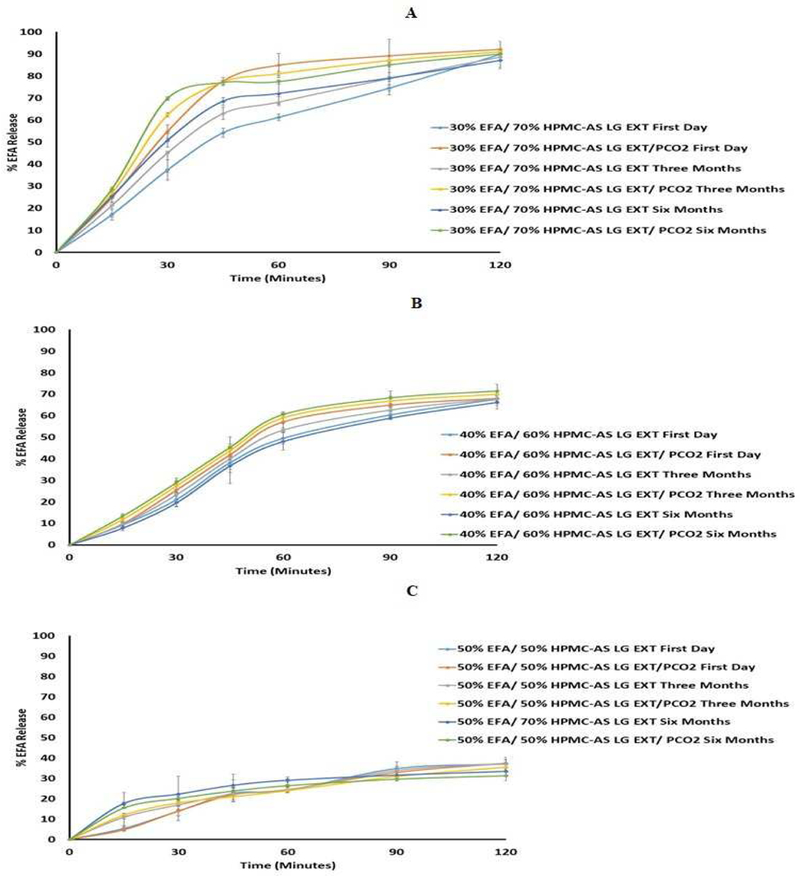
In-Vitro release profile of extrudates with and without P-CO2 (A USP dissolution apparatus II, 50 rpm, pH 6.8 phosphate buffer at 37 ± 0.5 °C for 2 h) stored at 25°C/60% RH. (A): formulation 30 % Extrudate (30% EFA / 70% HPMC-AS LG), (B): formulation 40 % Extrudate (40% EFA / 60% HPMC-AS LG), and (C): formulation 50 % Extrudate (50% EFA / 50% HPMC-AS LG)
