Figure 4.
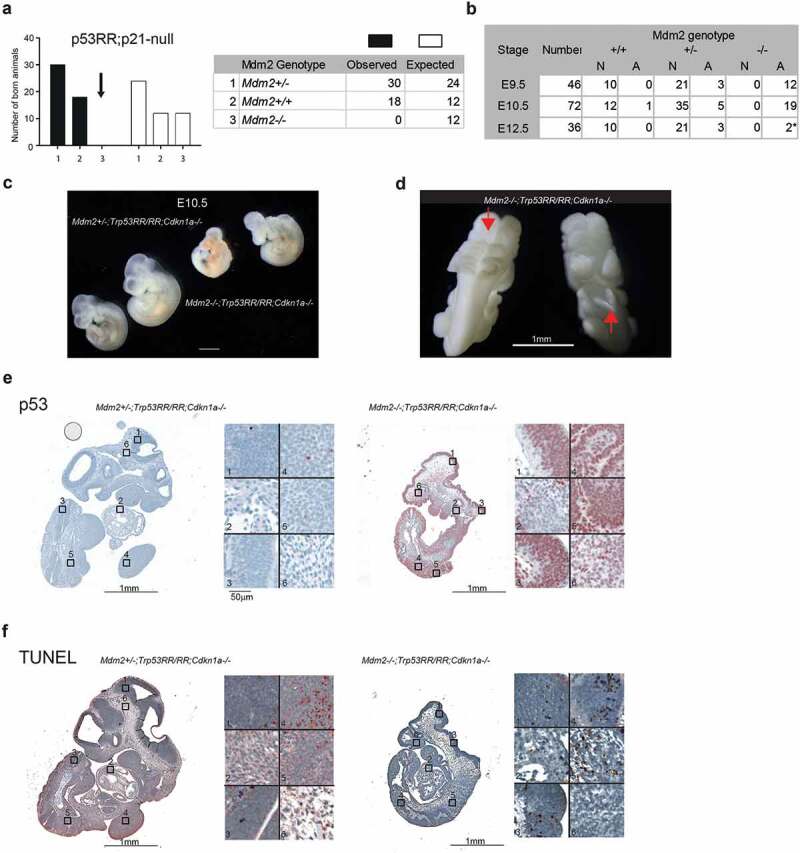
Genetic ablation of Cdkn1a fails to rescue embryonic lethality. (a) Observed and expected genotype distribution of newborn offspring from matings of Mdm2Δ7−9/Δ7−9;Cdkn1a–/–;Trp53RR/RR mice (total number of pups n = 48; contingency test P = 0.001). (b) Phenotype analysis of Cdkn1a–/–;Trp53RR/RR embryos with different Mdm2 genotypes shows the onset of development defects at the same stage (E9.5 – E10.5) as in Mdm2Δ7−9/Δ7−9; Trp53RR/RR embryos. N – normal, A – abnormal morphology. Asterisk – embryos were partially resorbed. (c) Representative images of Cdkn1a–/–;Trp53RR/RR embryos with hetero- and homozygous Mdm2 deletion at stage E10.5 (TS17-18) show same phenotypic abnormalities as in Mdm2Δ7−9/Δ7−9;Trp53RR/RR embryos. (d) Representative image of an Mdm2Δ7−9/Δ7−9;Cdkn1a–/–;Trp53RR/RR embryo shows neural tube closure defects (arrows) in cranial (left) and caudal (right) parts of the embryo. (e) Representative IHC staining of E10.5 embryos for p53 shows strong accumulation of p53 protein in Mdm2Δ7−9/Δ7−9;Cdkn1a–/–;Trp53RR/RR samples. Right panel: different tissues shown at high magnification (1,3 – neuroepithelial, 2 – heart, 4,5 – somites/limb bud, 6 – mesenchyme). (e) TUNEL assay in a serial section from the same samples as in (E). Note the enhanced apoptosis in neuroepithelial tissues and somites of Mdm2Δ7−9/Δ7−9;Cdkn1a–/–;Trp53RR/RR embryos.
