Figure 2.
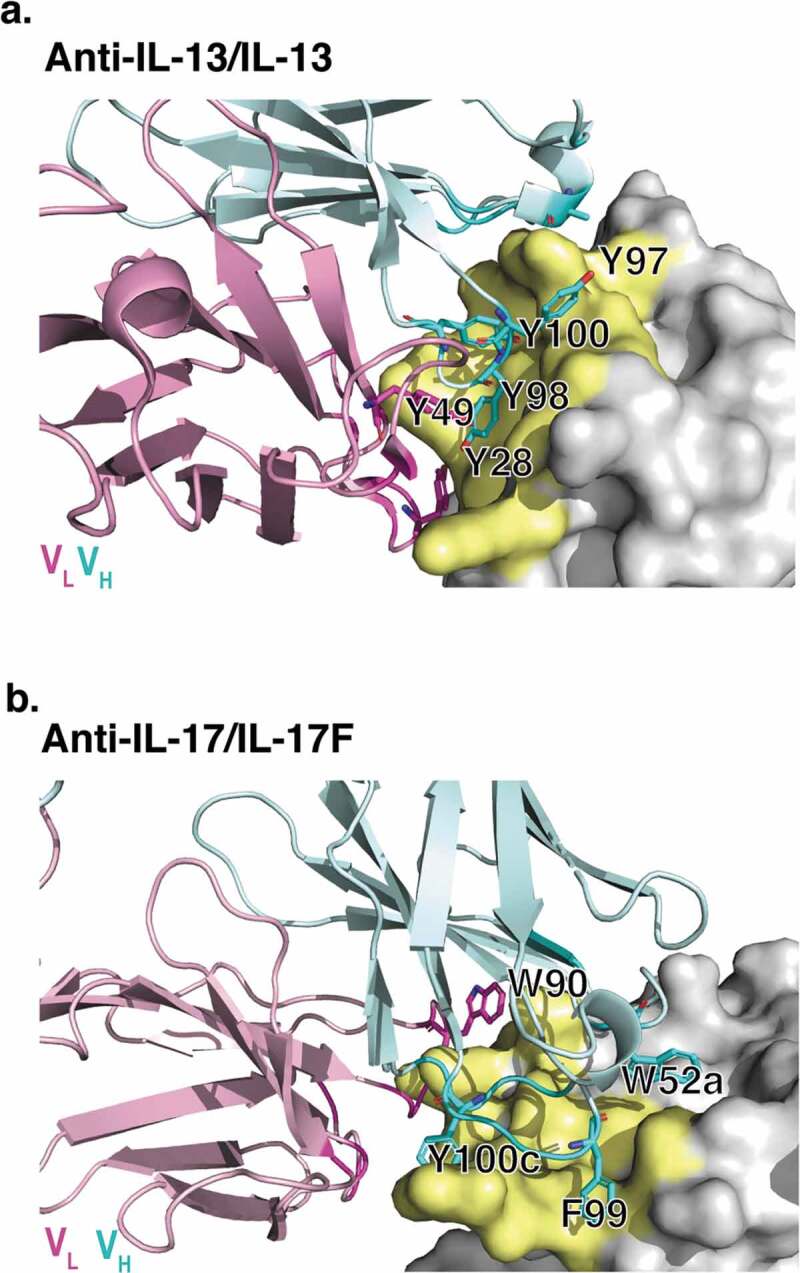
X-ray crystallographic structures of anti-IL-13 (lebrikizumab)40 and anti-IL-17 (MCAF5352A41) antibody Fabs complexed with their respective ligands displaying aromatic residues chosen for mutational studies. (a) View of anti-IL-13/IL-13 interface depicting important viscosity reducing residues (PDB 4I77).40 The anti-IL13 VH is cyan and VL is light pink, with dark cyan and magenta side chains within 4 Å of IL-13. IL-13 is gray with yellow epitope within 4 Å of anti-IL-13. (b) View of anti-IL-17/IL-17F interface depicting important residues (PDB 6PPG). Anti-IL-17F VH is cyan and VL is light pink, with dark cyan and magenta side chains within 4 Å of IL-17. IL-17F is gray with yellow epitope within 4 Å of anti-IL-17F. See the Supplementary Materials for the sequences for the variable domains for the anti-IL-13 (Figure S6) and anti-IL17 (Figure S7) antibodies.
