In a retrospective cohort study of 170 solid organ transplant recipients who completed treatment for an episode of cytomegalovirus disease, absolute lymphocyte count within a week of cessation of primary treatment emerged as an independent predictor of relapse.
Keywords: recurrent cytomegalovirus disease, transplant, lymphocyte count, lymphopenia
Abstract
Background
Recurrent cytomegalovirus (CMV) disease in solid organ transplant recipients frequently occurs despite effective antiviral therapy. We previously demonstrated that patients with lymphopenia before liver transplantation are more likely to develop posttransplant infectious complications including CMV. The aim of this study was to explore absolute lymphocyte count (ALC) as a predictor of relapse following treatment for CMV disease.
Methods
We performed a retrospective cohort study of heart, liver, and kidney transplant recipients treated for an episode of CMV disease. Our primary outcome was time to relapse of CMV within 6 months. Data on potential predictors of relapse including ALC were collected at the time of CMV treatment completion. Univariate and multivariate hazard ratios (HRs) were calculated with a Cox model. Multiple imputation was used to complete the data.
Results
Relapse occurred in 33 of 170 participants (19.4%). Mean ALC in relapse-free patients was 1.08 ± 0.69 vs 0.73 ± 0.42 × 103 cells/μL in those who relapsed, corresponding to an unadjusted hazard ratio of 1.11 (95% confidence interval, 1.03–1.21; P = .009, n = 133) for every decrease of 100 cells/μL. After adjusting for potential confounders, the association between ALC and relapse remained significant (HR, 1.11 [1.03–1.20]; P = .009).
Conclusions
Low ALC at the time of CMV treatment completion was a strong independent predictor for recurrent CMV disease. This finding is biologically plausible given the known importance of T-cell immunity in maintaining CMV latency. Future studies should consider this inexpensive, readily available marker of host immunity.
Cytomegalovirus (CMV) remains a major contributor to morbidity, graft loss, and mortality following solid organ transplantation (SOT). Because viral DNA persists lifelong, constant immune surveillance, predominantly by T-lymphocytes, is needed to prevent viral replication [1, 2]. In transplant recipients, the use of antilymphocyte-based immunosuppression affects antiviral immune control, which can result in CMV reactivation and diverse clinical manifestations ranging from a febrile illness to severe end-organ disease [3], as well as indirect effects including rejection, secondary infections, malignancy, and overall decreased graft and patient survival [4, 5]. Effective antiviral therapy with intravenous ganciclovir and oral valganciclovir has had a dramatic impact on CMV disease in SOT recipients; however, the use of these drugs can be limited by cost and side effects such as bone marrow suppression, which occurs in 10%–30% of patients [6].
Up to 30% of SOT recipients treated for CMV disease relapse. Despite identification of risk factors and advancements in the understanding of CMV-specific immune responses, relapse rates have remained consistent over time [7–10]. Known risk factors for relapse relate to the extent of initial disease and the degree of ongoing immunosuppression, and include primary CMV infection (ie, a CMV-seronegative recipient receiving an organ from a seropositive donor, or acquiring CMV from another source posttransplant), high initial viral load, prolonged viremia, persistent viremia at treatment completion, multiorgan disease, CMV pneumonitis, treatment for rejection, cadaveric kidney/kidney–pancreas/thoracic organ transplantation, and “extensive disease” in patients with gastrointestinal CMV [7–9, 11–16]. Quantifying the level of immune activity or “net state of immunosuppression” within an individual transplant recipient is challenging, but likely represents the most significant determinant of CMV reactivation. Currently, there is no reliable way to predict which patients will maintain immune control of CMV infection following treatment, and which patients will go on to relapse.
The absolute lymphocyte count (ALC) is a simple, inexpensive, readily, and reliably measurable value. In patients with human immunodeficiency virus infection, the CD4 count is an established biomarker that predicts the development of opportunistic infections including CMV [17]. In bone marrow transplant recipients, lymphopenia at the time of CMV infection has been associated with increased mortality [18, 19]. In SOT recipients, several studies have associated pre- or posttransplant ALC [20–22], as well as specific lymphocyte subsets with the development of CMV and other infectious complications following transplantation [23, 24]. Finally, expansion of the CD8+ T-lymphocyte pool has been associated with resolution of CMV infection in kidney transplant recipients [25]. These findings led us to hypothesize that a low ALC at the end of treatment of an episode of CMV disease in SOT recipients may increase the risk of subsequent relapse. The aim of this study was to explore potential predictors for recurrent CMV disease with a focus on the effect of ALC in a retrospective cohort of heart, liver, and kidney transplant recipients who completed treatment for an episode of CMV disease.
METHODS
Study Design and Population
SOT recipients who developed an episode of CMV infection at Tufts Medical Center between 1995 and 2016 were eligible for inclusion in our study. Participants were identified by searching electronic medical records for positive CMV test results, including viral cultures, histopathology, and viral load testing. Patients were excluded if they (1) did not complete a course of antiviral therapy; (2) died, were lost to follow-up, or experienced graft failure during treatment or within 2 weeks following treatment completion; or (3) did not have sufficient clinical information available in their medical record. The Tufts Medical Center Institutional Review Board approved this study; informed consent was not required given its retrospective nature and minimal risk. Clinical data were obtained from hospital medical records. A panel of 3 transplant infectious disease physicians blinded to ALC assessed all cases to ensure they met inclusion/exclusion criteria and to determine if they experienced relapse.
Immunosuppression and Rejection Protocols
Patients received maintenance immunosuppression with a calcineurin inhibitor, an antimetabolite, and prednisone, per our institutional protocols [26]. There was a transition around the year 2000 from cyclosporine/azathioprine without induction to tacrolimus/mycophenolate with antilymphocyte induction, routinely for kidney transplant recipients and for heart/liver recipients with renal impairment. Agents used included antithymocyte globulin, OKT3 (muromonab-CD3), daclizumab, and basiliximab.
Rejection was biopsy proven and, if moderate/severe, was treated with intravenous corticosteroids, typically methylprednisolone 1 g daily for 3–5 days. Patients were defined as having received steroids for rejection if their baseline dose was increased by ≥3-fold. Patients with steroid-refractory cell-mediated rejection usually received antithymocyte globulin, and those with antibody-mediated rejection were treated with rituximab, plasmapheresis, intravenous immunoglobulin, bortezomib, or, occasionally, photopheresis.
CMV Prophylaxis, Diagnosis, and Treatment
All CMV-seropositive recipients or those receiving an organ from a CMV-seropositive donor received routine primary prophylaxis for 3–6 months following transplantation with intravenous ganciclovir 5 mg/kg once daily, oral ganciclovir 1000 mg 3 times daily, or oral valganciclovir 900 mg once daily, with doses adjusted for renal impairment according to the package insert (Genentech, San Francisco, California). Additionally, donor-seropositive, recipient-seronegative patients received 7 doses of CMV immune globulin (CMVIG) until 2002, except heart transplant recipients who continued to receive it until 2014. Many patients who experienced rejection during their primary CMV prophylaxis had their duration of prophylaxis extended, and some were recommenced on primary prophylaxis following their rejection episode if it occurred after completion of prophylaxis. Routine viral load surveillance after prophylaxis was not performed.
CMV infection was defined as a positive test for CMV at any site. Viral load, viral culture, and histopathologic testing were performed using standard techniques. Over the 20-year study period, 3 viral load assays were used: (1) the Hybrid Capture CMV DNA assay version 2.0 (Digene, Silver Spring, Maryland; now Qiagen), a whole blood assay with a detection range of 2.1 to >830 pg/mL (1997–2008); (2) a whole blood assay performed by Quest Diagnostics (Chantilly, Virginia), detection range of 200 to >200000 copies/mL (2008–2011); and (3) a plasma assay (Focus Diagnostics “Simplexa” kit) with a detection range of 1000 (values <1000 can be detected but not quantified) to 500000 copies/mL (2011–present). To collapse virologic data into a single categorical variable, patients with peak values over the midpoint of the range of each assay were classified as having a high viral load.
CMV end-organ disease required laboratory confirmation of CMV plus clinical evidence of organ dysfunction, categorized as proven (positive pathology or cultures at a nonblood site with attributable symptoms), probable (DNAemia plus attributable symptoms), or possible (DNAemia with clinical symptoms suggestive of end-organ involvement but a potential alternative diagnosis). Patients with CMV syndrome had detection of CMV in blood plus ≥2 of the following: fever for ≥2 days, new/increased malaise, neutropenia (<1.5 × 103/μL) or thrombocytopenia (<150 × 103/μL), elevated aspartate aminotransferase/alanine aminotransferase (>2 times the upper limit of normal, nonliver recipients only).
Standard treatment of CMV infection was with intravenous ganciclovir 5 mg/kg twice daily or oral valganciclovir 900 mg twice daily, with doses adjusted for renal impairment according to the package insert. Duration of therapy was individualized and determined by clinical and virologic endpoints but was generally at least 2–3 weeks, with most patients treated until resolution of DNAemia. If ganciclovir resistance was suspected, alternative antiviral agents were considered and genotypic testing performed. Use of secondary prophylaxis and frequency of viral load monitoring following treatment was determined by treating clinicians.
Relapse
The primary outcome was time to relapse of CMV infection, defined as CMV infection or disease that occurred within 6 months following the completion of successful treatment for an initial episode of CMV disease. Patients were considered to have relapsed if (1) they became symptomatic with any positive test for CMV; (2) were asymptomatic but had a single positive viral load test above the lower limit of detection of the assay; or (3) were asymptomatic but had ≥2 consecutively positive tests <1000 copies/mL (assay 3). Asymptomatic patients with a single positive but unquantifiable viral load were not counted as relapses. This definition was adapted from previous studies and modified for our patient population and local diagnostic testing [6, 13, 26–28].
Covariates
Data were collected on variables available at the time of CMV treatment completion that could be associated with the development of recurrent CMV disease and/or lymphocyte count, including demographics, organ received, CMV serostatus at time of transplant, immunosuppression, clinical details of CMV episode, and treatment information. Laboratory results, including lymphocyte counts, were obtained as close as possible to the last day of completion of CMV antiviral therapy. Values unavailable within 1 week were considered missing.
Statistical Analyses
Descriptive statistics were calculated, with categorical data reported as counts and percentages, continuous data as mean ± standard deviations if normally distributed, and medians with ranges if nonnormal. P values of <.05 were considered statistically significant. Multiple imputation was performed to complete missing data, using the “mice” package in R software version 2.30. Censoring occurred at the time of relapse, death, graft failure, loss to follow-up, or 6 months following treatment completion.
Kaplan-Meier survival curves representing time to relapse were compared using the log-rank test. Univariate hazard ratios (HRs) calculated using a Cox model were used to evaluate predictors of relapse. A multivariable Cox model was developed using a combined approach of a priori variable selection based on clinical reasoning as well as statistical selection. Variables thought most likely to influence risk of relapse were selected based on clinical experience and the literature by a panel of experts blinded to ALC and outcome. Events occurring after CMV treatment completion were not included. Model diagnostics were performed to ensure assumptions were met, and multiple sensitivity analyses were conducted to confirm our findings (Supplementary Materials). All analyses were performed with R version 3.4.1 software (RStudio version 1.0.153).
RESULTS
One hundred and seventy SOT recipients (79 kidney, 52 heart, 34 liver and 5 liver-kidney) who completed treatment for an episode of CMV disease were included in our final cohort, after removing 66 patients who met exclusion criteria [26]. Median age at CMV onset was 55 years; 65% were male; and median time from transplant to CMV diagnosis was 7 months. Pretransplant CMV serostatus was available for all recipients and 168 donors; 86 (51%) were donor positive/recipient negative, 52 (31%) were donor and recipient positive, 24 (14%) were donor negative/recipient positive, and 6 (4%) were donor and recipient negative. A total of 120 patients (71%) had evidence of end-organ disease, most commonly in the gastrointestinal tract. Forty-four (26%) had CMV syndrome without localizing symptoms and 6 (4%) had asymptomatic viremia. Median treatment duration was 4 weeks. Five patients had clinically suspected ganciclovir resistance, but only 1 had a confirmed UL97 mutation (this patient responded to high-dose ganciclovir), and another was treated empirically with foscarnet. Relapse occurred in 33 patients within 6 months of completion of antiviral therapy. Thirteen patients were censored before 6 months, with 10 deaths, 2 lost to follow-up, and 1 graft failure. At the time of relapse, 13 patients had CMV syndrome, 13 had proven/suspected end-organ disease, 7 had asymptomatic viremia, and 26 were retreated with antivirals.
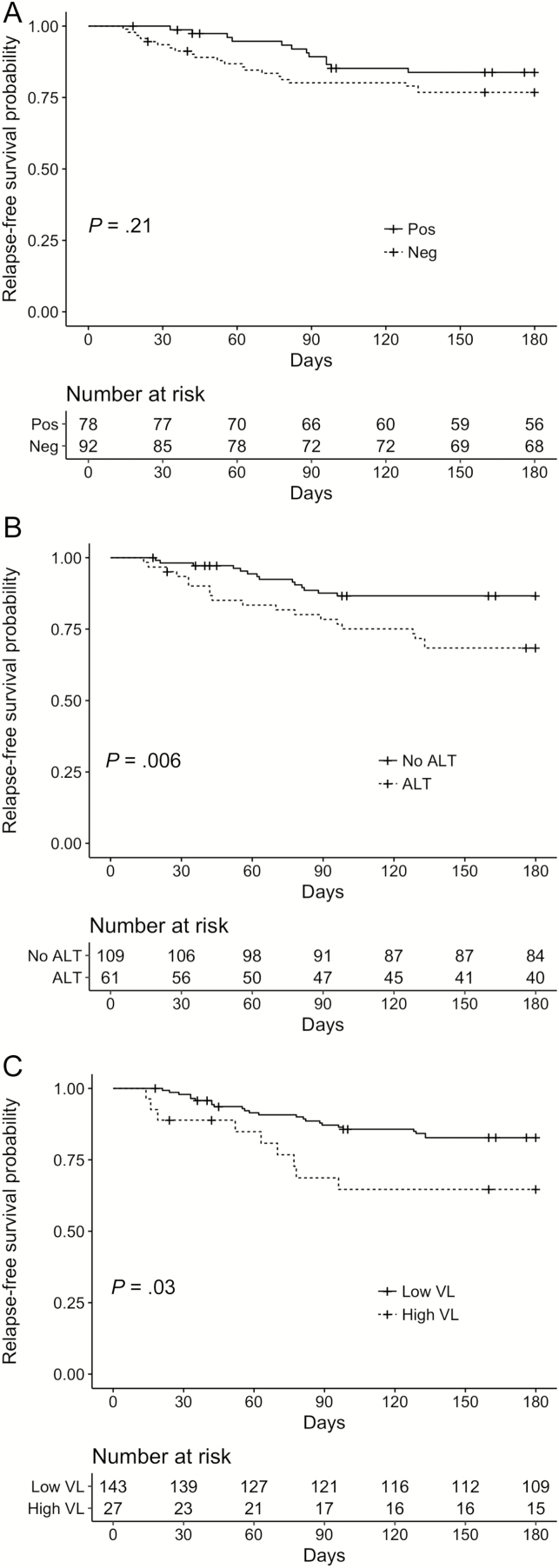
Baseline characteristics stratified by relapse status are shown in Table 1, with univariate HR for CMV relapse. Factors significantly associated with relapse included older age, later calendar year, living unrelated kidney transplant, use of an antilymphocyte agent within a year, high peak viral load, longer treatment duration, use of adjunctive CMVIG, and lower ALC at treatment completion. Patients who were treated for rejection with intravenous corticosteroids within the year leading up to the end of CMV treatment were significantly less likely to relapse. The relapse rate among donor-positive/recipient-seronegative patients was 24%, compared with 14% in other serogroups. Kaplan-Meier plots showing unadjusted relapse-free survival probabilities stratified by receipt of antilymphocyte therapy within 1 year prior to treatment completion, recipient CMV serostatus, and peak viral load are shown in Figure 1.
Table 1.
Characteristics of Patients With Relapsing Cytomegalovirus Infection Within 6 Months Compared to Those Without Relapse, and Unadjusted Hazard Ratios for Relapse
| Characteristic | Overall (N = 170) |
No Relapse (n = 137) | Relapse (n = 33) |
Unadjusted HR (95% CI) |
P Value |
|---|---|---|---|---|---|
| Age at CMV onset, y, median (range) | 54.5 (14–80) | 53 (14–79) | 60 (18–80) | 1.03 (1–1.06) | .05 |
| Male sex | 111 (65) | 90 (66) | 21 (64) | 0.92 (.45–1.88) | .83 |
| Race, white (vs nonwhite) | 127 (75) | 104 (76) | 23 (70) | 0.78 (.37–1.64) | .51 |
| Year of CMV infection, median (range) | 2007 (1995–2016) | 2007 (1995–2016) | 2009 (1995–2015) | 1.08 (1.01–1.16) | .03 |
| Transplanted organ | |||||
| Heart | 52 (31) | 46 (34) | 6 (18) | Ref | |
| Liver (including liver–kidney) | 34 (20) | 34 (25) | 5 (15) | 1.08 (.33–3.55) | .89 |
| Kidney (living unrelated donor) | 16 (9) | 8 (6) | 8 (24) | 5.33 (1.85–15.37) | .002 |
| Kidney (deceased donor) | 48 (28) | 10 (30) | 10 (30) | 1.77 (.64–4.86) | .27 |
| Kidney (living related donor) | 15 (9) | 4 (12) | 4 (12) | 2.35 (.66–8.34) | .19 |
| Previous transplant | 16 (9) | 13 (10) | 3 (9) | 0.99 (.30–3.26) | .99 |
| Recipient CMV seronegative pretransplant | 92 (54) | 71 (51) | 21 (64) | 1.57 (.77–3.20) | .21 |
| Any antilymphocyte agent within 1 y before CMV treatment completion | 61 (36) | 42 (31) | 19 (58) | 2.61 (1.31–5.21) | .006 |
| Type of antilymphocyte agent | |||||
| None | 109 (64) | 95 (69) | 14 (42) | Ref | |
| Basiliximab/daclizumab | 13 (8) | 7 (5) | 6 (18) | 4.47 (1.72–11.64) | .002 |
| ATG/OKT3 | 48 (28.2) | 35 (26) | 13 (39) | 2.19 (1.03–4.67) | .04 |
| No. of immunosuppressive drugs | |||||
| 1 | 13 (8) | 9 (7) | 4 (10) | Ref | |
| 2 | 85 (50) | 64 (49) | 21 (54) | 0.65 (.22–1.93) | .44 |
| 3 | 72 (42) | 58 (44) | 14 (36) | 0.53 (.17–1.66) | .28 |
| Steroid-treated rejection | |||||
| Within 1 y before treatment completion | 38 (22) | 36 (26) | 2 (6) | 0.21 (.05–.87) | .03 |
| Following treatment completion to censoring | 10 (6) | 6 (4) | 4 (12) | 2.2 (.78–6.30) | .14 |
| Time from transplant to CMV onset, wk, median (range) | 31 (4–1131) | 33 (6–1131) | 29 (4–755) | 1.00 (.99–1.01) | .91 |
| Clinical details | |||||
| Time of symptoms prior to proven onset, d, median (range) | 11.5 (0–365) | 12 (0–209) | 11 (0–365) | 1.00 (.99–1.01) | .56 |
| End-organ disease | 120 (71) | 96 (70) | 24 (73) | 1.06 (.49–2.28) | .88 |
| Proven/probable site | 105 (62) | 84 (61) | 21 (64) | 1.04 (.51–2.12) | .91 |
| High viral load | 27 (16) | 18 (13) | 9 (27) | 2.40 (1.12–5.17) | .03 |
| Admitted for CMV | 134 (79) | 107 (78) | 27 (82) | 1.26 (.52–3.04) | .61 |
| Length of stay, d, median (range) (n = 134) | 6 (1–46) | 5 (1–46) | 7 (2–39) | 1.03 (1.00–1.08) | .07 |
| Length of antiviral therapy, d, median (range) | 28.5 (3–201) | 26 (3–201) | 40 (3–145) | 1.01 (1.00–1.02) | .004 |
| Treatment type | |||||
| Oral only | 72 (42) | 60 (44) | 12 (36) | Ref | |
| IV and oral | 64 (38) | 50 (37) | 14 (42) | 1.36 (.63–2.93) | .44 |
| IV only | 34 (20) | 27 (20) | 7 (21) | 1.28 (.50–3.26) | .60 |
| Adjunctive CMV immune globulin | 17 (10) | 8 (6) | 9 (27) | 5.16 (2.39–11.14) | <.001 |
| Laboratory results, mean ± SD | |||||
| Total WBC count, ×103 cells/μL (n = 140) | 4.62 ± 2.57 | 4.70 ± 2.66 | 4.33 ± 2.21 | 0.94 (.80–1.10)a | .44 |
| ALC, ×103 cells/μL (n = 133) | 1.01 ± 0.66 | 1.08 ± 0.69 | 0.73 ± 0.42 | 1.11 (1.03–1.21)b | .009 |
| ANC, ×103 cells/μL (n = 133) | 3.01 ± 1.93 | 2.97 ± 1.89 | 3.19 ± 2.10 | 1.04 (.86–1.25)a | .70 |
| CKD-EPI eGFR, mL/min/1.73 m2 (n = 140) | 57 ± 25 | 57 ± 25 | 55 ± 25 | 1.00 (.98–1.01)a | .56 |
| Received secondary prophylaxis | 120 (71) | 97 (71) | 23 (70) | 0.91 (.43–1.92) | .81 |
| Duration of secondary prophylaxis, d, median (range) (n = 120) | 60.5 (5–180) | 66 (5–180) | 55 (5–105) | 0.98 (.97–.99) | .01 |
Data are presented as No. (%) and refer to the time of CMV treatment completion, unless otherwise stated.
Abbreviations: ATG, antithymocyte globulin; ALC, absolute lymphocyte count; ANC, absolute neutrophil count; CI, confidence interval; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; CMV, cytomegalovirus; eGFR, estimated glomerular filtration rate; HR, hazard ratio; IV, intravenous; OKT3, muromonab-CD3; SD, standard deviation; WBC, white blood cell.
aHRs displayed to reflect effect per each 1-unit increase in value.
bHRs displayed to reflect effect per each incremental decrease of 100 cells/μL in the ALC.
Figure 1.
Unadjusted Kaplan-Meier estimates of relapse-free survival stratified by recipient cytomegalovirus (CMV) serostatus at the time of transplantation (A), receipt of antilymphocyte therapy (ALT) within 1 year prior to the completion of CMV treatment (B), and peak viral load (VL) (C). P values refer to log-rank test results (n = 170).
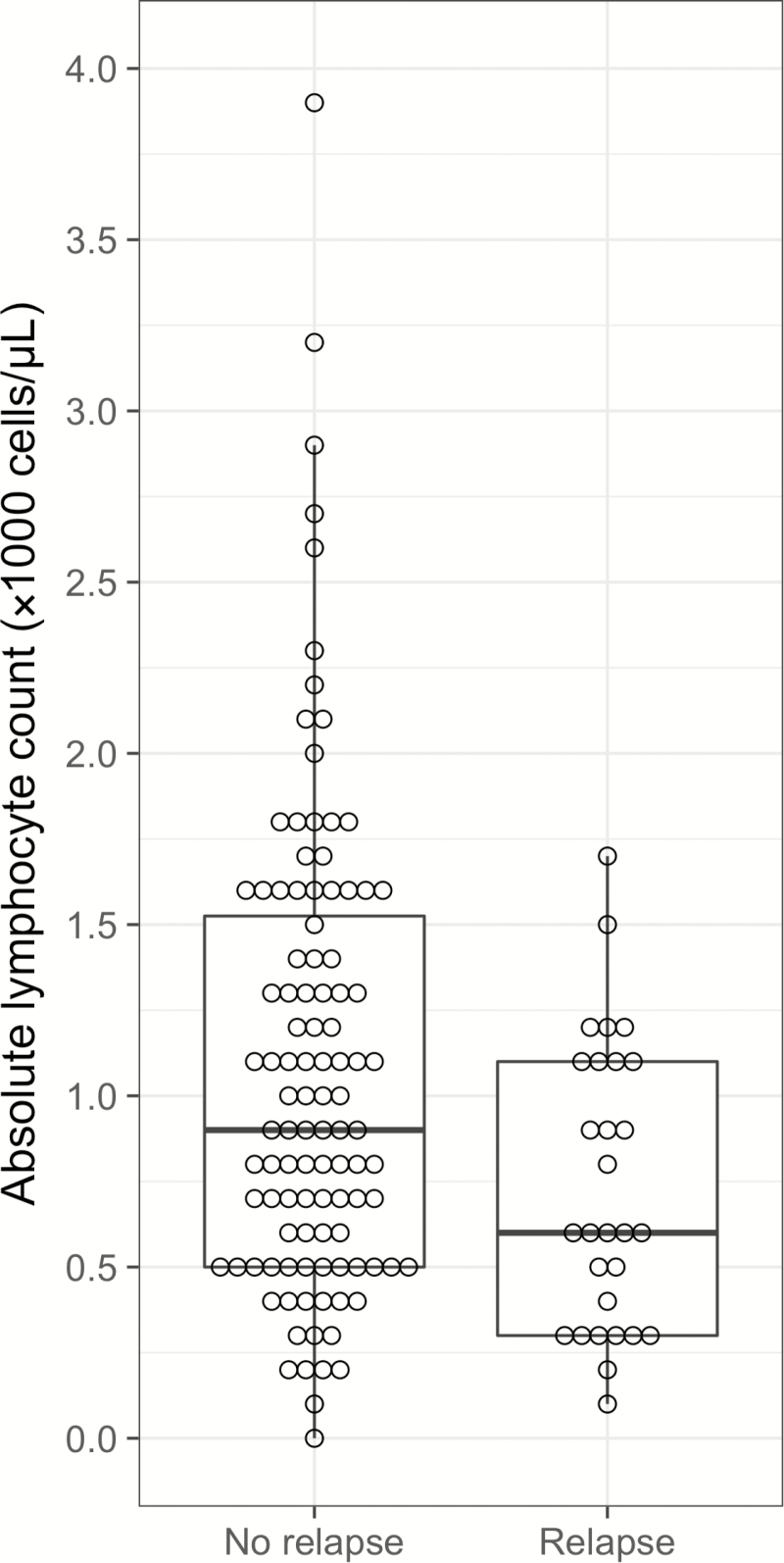
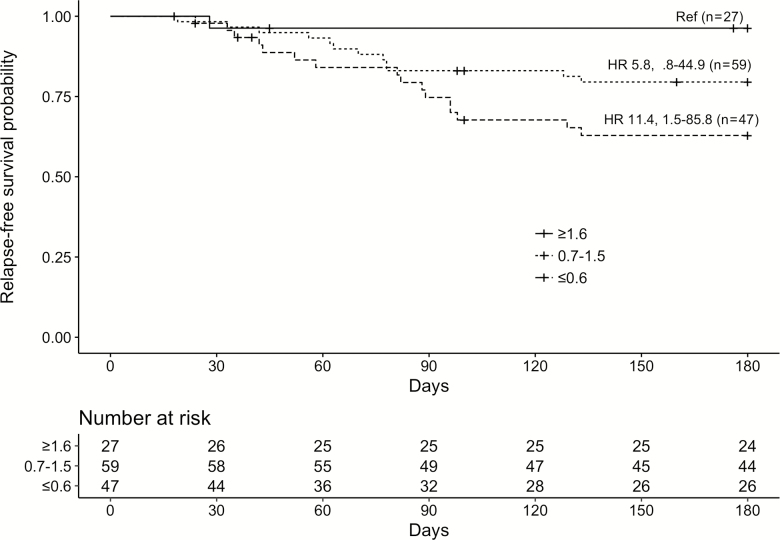
Lymphocyte counts within 1 week of treatment completion were available for 133 patients. Mean ALC in relapse-free patients was 1.08 ± 0.69 × 103 cells/μL vs 0.73 ± 0.42 × 103 cells/μL in those who relapsed (Figure 2). Unadjusted, and based on patients who had complete data only, the HR for every decrease of 100 cells/μL in the ALC was 1.11 (95% confidence interval [CI], 1.03–1.21; P = .009), indicating an increasing likelihood of relapse with lower ALC values. None of the 15 patients with an ALC ≥1.8 × 103 cells/μL relapsed. Figure 3 illustrates the relapse-free survival probabilities of patients stratified by their baseline lymphocyte count, again demonstrating the increased likelihood of relapse with lower ALC. The independent relationship between low ALC and relapse persisted even after adjusting for several potential confounders in our primary multivariate analysis (Table 2), which used multiple imputation (based on 26 covariates and 5 imputations) to complete the missing lymphocyte count data and accounted for use of an antilymphocyte agent, higher peak viral load, and negative recipient CMV serostatus (adjusted HR, 1.11 [95% CI, 1.03–1.20] for every decrease of 100 cells/μL; P = .009). Consistent results were obtained across multiple sensitivity analyses, with hazard ratios for ALC remaining stable and statistically significant (Supplementary Materials). No evidence of confounding was seen despite repeating analyses controlling for all measured covariates, including duration and type of antiviral therapy. Total white blood cell count and neutrophil count were not significantly associated with relapse (Table 1).
Figure 2.
Distribution of absolute lymphocyte counts at time of cytomegalovirus (CMV) treatment completion in patients who did (n = 29) and did not (n = 104) experience CMV relapse.
Figure 3.
Unadjusted Kaplan-Meier estimates of relapse-free survival stratified by absolute lymphocyte count (×1000 cells/μL) at the time of treatment completion (n = 133). Hazard ratios (HRs) for relapse for each strata with 95% confidence intervals are displayed.
Table 2.
Results of Multivariable Analysis Demonstrating Adjusted Hazard Ratios for Relapse of Cytomegalovirus Infection
| Variable | Adjusted HR (95% CI) |
P Value |
|---|---|---|
| Antilymphocyte therapy | 2.51 (1.21–5.20) | .01 |
| Recipient CMV seronegative | 1.89 (.89–4.01) | .10 |
| High peak viral load | 2.67 (1.21–5.89) | .02 |
| Decrease in ALC (per 100 cells/μL increment) | 1.11 (1.03–1.20) | .009 |
Missing lymphocyte count data completed using multiple imputation.
Abbreviations: ALC, absolute lymphocyte count; CI, confidence interval; CMV, cytomegalovirus; HR, hazard ratio.
Use of adjunctive CMVIG for treatment of CMV disease was strongly associated with relapse (HR, 5.16 [95% CI, 2.39–11.14]; P < .001). However, patients receiving CMVIG were significantly more likely to have a longer length of stay (14 ± 13 days vs 5 ± 5 days; P < .01), to receive longer courses of therapy (54 ± 49 vs 37 ± 28 days; P = .04), and to receive intravenous therapy (100% vs 53%; P < .01), suggesting that this association may be due to confounding by indication.
DISCUSSION
Despite advances in SOT, the management of CMV disease, and an improved understanding of the immunobiology of CMV infection, rates of recurrent disease following treatment of CMV infection remain consistently high. The immune investment in CMV control is significant and multifaceted, and an effective response, predominantly mediated by T lymphocytes, is essential for long-term viral control. While pre- and posttransplant lymphopenia have been associated with infectious outcomes including development of CMV disease in liver and kidney transplant recipients, respectively [20–22], ALC has not previously been explored as a predictor of relapse following CMV infection. In our study, lymphocyte count emerged as a strong, independent predictor of relapse, with patients having a lower ALC at the end of treatment being significantly more likely to develop recurrent disease. This finding is biologically plausible in the context of our understanding of the immunology of CMV latency [29], and could help identify patients at high risk of recurrent CMV disease.
Our study is the first to describe an association between ALC at the time of CMV treatment completion and the subsequent development of recurrent CMV disease. Strengths include the relatively large and diverse cohort, described in detail and analyzed using robust statistical methodology, with consistent results obtained across different analyses. Many of our findings were similar to those of prior studies [8, 12–15, 21, 22, 30, 31], although we defined immunosuppression differently, focusing on receipt of antilymphocyte agents within 1 year prior to CMV treatment completion. Our findings suggest that antilymphocyte therapy increases the risk of recurrence by other mechanisms beyond just inducing lymphopenia, and that lymphopenia is an independent predictor of relapse regardless of its etiology. We have previously demonstrated that use of secondary prophylaxis following treatment completion has no overall long-term benefit but is protective while patients continue to receive the antiviral drug [26]. ALC could be used to help target secondary prophylaxis to those at the highest risk of relapse.
There are some limitations that should be considered when interpreting our results. Ours was a single-center, retrospective study with statistical power limited by the number of cases and outcomes, which meant we could not control for all potential confounders in our multivariable models simultaneously. There were changes in CMV diagnostics, treatment, and immunosuppression protocols over the 20-year study period. Although 20% of patients were missing ALC values, we were able to address this using multiple imputation, avoiding the introduction of bias that can arise from excluding patients with missing data [32]. Results of complete case and imputed analyses were similar. ALC was assessed at one time point only, so we could not account for fluctuations in ALC over time, which may also influence the risk of relapse. We were unable to explore reasons for lymphopenia, which could include degree of iatrogenic immunosuppression, residual bone marrow involvement of CMV, or another cause. Additionally, during the course of our study, 3 different viral load assays were used precluding direct comparison of values. We addressed this by categorizing patients as having a high or low viral load, an oversimplification that unfortunately resulted in some loss of power for this variable. Finally, frequency of viral load testing following treatment completion was not standardized, so it is possible some cases of asymptomatic viremia were not detected. Despite these limitations, our findings were internally consistent as well as supportive of other similar studies [20–24].
Lymphocyte function as well as absolute number is likely to be important in the development of recurrent CMV disease, though measuring this is more complex. Promising new immunologic assays that can assess patient-specific immune responses to CMV, such as the QuantiFERON-CMV, are becoming available, and emerging data on the performance of these tests are encouraging [33–35]. These tests may facilitate risk stratification of patients for development of initial CMV disease as well as for recurrence [36]. However, comprehensive clinical studies informing how and when to use these assays, how to best integrate them into clinical practice, and their incremental value compared to simpler biomarkers such as ALC, are lacking. In addition, these assays are expensive and not widely available, particularly in the United States.
In conclusion, our findings suggest that ALC may be a novel, strong, simple, independent predictor of recurrent CMV disease. This has potential clinical utility and is an important step toward the ultimate goal of accurate and reliable individualized risk prediction, which could help facilitate the targeted use of interventions such as longer courses of antiviral therapy, secondary prophylaxis, or more intensive follow-up. Further studies are required to validate this finding and to assess its generalizability in other patient populations, with the ongoing aim of reducing the morbidity of CMV disease and improving long-term posttransplant outcomes.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors acknowledge the assistance of Emily Miller, Nitin Jethmalani, Kevin Lindell, and Shelley Bame-Aldred for help with data collection, and Alejandro Moreno-Koehler, Lori-Lyn Price, and Angie Rodday for statistical and data management support.
Financial support. This work was supported by the Tufts Medical Center Division of Geographic Medicine and Infectious Disease Francis P. Tally MD Fellowship; the National Institutes of Health Clinical and Translational Science Award (grant number UL1TR001064); the Monash University Sir James McNeill Postgraduate Research Scholarship (an Australian Government Research Training Program Scholarship); and the National Health and Medical Research Council of Australia (grant number GNT1150351).
Potential conflicts of interest. D. R. S. has served on advisory boards for Merck, Shire, Summit, Chimerix, and Seres Therapeutics and has received grants from Merck, Summit, Actelion, Tetraphase, and Seres Therapeutics. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Crough T, Khanna R. Immunobiology of human cytomegalovirus: from bench to bedside. Clin Microbiol Rev 2009; 22:76–98, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goodrum F. Human cytomegalovirus latency: approaching the Gordian knot. Annu Rev Virol 2016; 3:333–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Razonable RR, Humar A; AST Infectious Diseases Community of Practice Cytomegalovirus in solid organ transplantation. Am J Transplant 2013; 13:93–106. [DOI] [PubMed] [Google Scholar]
- 4. Snydman DR, Limaye AP, Potena L, Zamora MR. Update and review: state-of-the-art management of cytomegalovirus infection and disease following thoracic organ transplantation. Transplant Proc 2011; 43:S1–17. [DOI] [PubMed] [Google Scholar]
- 5. Freeman RB., Jr The ‘indirect’ effects of cytomegalovirus infection. Am J Transplant 2009; 9:2453–8. [DOI] [PubMed] [Google Scholar]
- 6. Kotton CN, Kumar D, Caliendo AM, et al. ; Transplantation Society International CMV Consensus Group Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation 2013; 96:333–60. [DOI] [PubMed] [Google Scholar]
- 7. Natori Y, Humar A, Husain S, et al. . Recurrence of CMV infection and the effect of prolonged antivirals in organ transplant recipients. Transplantation 2017; 101:1449–54. [DOI] [PubMed] [Google Scholar]
- 8. Eid AJ, Arthurs SK, Deziel PJ, Wilhelm MP, Razonable RR. Clinical predictors of relapse after treatment of primary gastrointestinal cytomegalovirus disease in solid organ transplant recipients. Am J Transplant 2010; 10:157–61. [DOI] [PubMed] [Google Scholar]
- 9. Humar A, Uknis M, Carlone-Jambor C, Gruessner RW, Dunn DL, Matas A. Cytomegalovirus disease recurrence after ganciclovir treatment in kidney and kidney-pancreas transplant recipients. Transplantation 1999; 67:94–7. [DOI] [PubMed] [Google Scholar]
- 10. Sawyer MD, Mayoral JL, Gillingham KJ, Kramer MA, Dunn DL. Treatment of recurrent cytomegalovirus disease in patients receiving solid organ transplants. Arch Surg 1993; 128: 165–9. [DOI] [PubMed] [Google Scholar]
- 11. Asberg A, Humar A, Jardine AG, et al. ; VICTOR Study Group Long-term outcomes of CMV disease treatment with valganciclovir versus IV ganciclovir in solid organ transplant recipients. Am J Transplant 2009; 9:1205–13. [DOI] [PubMed] [Google Scholar]
- 12. Asberg A, Jardine AG, Bignamini AA, et al. ; VICTOR Study Group Effects of the intensity of immunosuppressive therapy on outcome of treatment for CMV disease in organ transplant recipients. Am J Transplant 2010; 10:1881–8. [DOI] [PubMed] [Google Scholar]
- 13. Falagas ME, Snydman DR, Griffith J, Werner BG, Freeman R, Rohrer R. Clinical and epidemiological predictors of recurrent cytomegalovirus disease in orthotopic liver transplant recipients. Boston Center for Liver Transplantation CMVIG Study Group. Clin Infect Dis 1997; 25:314–7. [DOI] [PubMed] [Google Scholar]
- 14. Helanterä I, Lautenschlager I, Koskinen P. The risk of cytomegalovirus recurrence after kidney transplantation. Transpl Int 2011; 24:1170–8. [DOI] [PubMed] [Google Scholar]
- 15. Helanterä I, Schachtner T, Hinrichs C, et al. . Current characteristics and outcome of cytomegalovirus infections after kidney transplantation. Transpl Infect Dis 2014; 16:568–77. [DOI] [PubMed] [Google Scholar]
- 16. Nafar M, Roshan A, Pour-Reza-Gholi F, et al. . Prevalence and risk factors of recurrent cytomegalovirus infection in kidney transplant recipients. Iran J Kidney Dis 2014; 8:231–5. [PubMed] [Google Scholar]
- 17. Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. Available at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. Accessed 23 February 2017. [Google Scholar]
- 18. Einsele H, Ehninger G, Steidle M, et al. . Lymphocytopenia as an unfavorable prognostic factor in patients with cytomegalovirus infection after bone marrow transplantation. Blood 1993; 82:1672–8. [PubMed] [Google Scholar]
- 19. Fries BC, Khaira D, Pepe MS, Torok-Storb B. Declining lymphocyte counts following cytomegalovirus (CMV) infection are associated with fatal CMV disease in bone marrow transplant patients. Exp Hematol 1993; 21:1387–92. [PubMed] [Google Scholar]
- 20. Corona-Nakamura AL, Monteon-Ramos FJ, Troyo-Sanroman R, Arias-Merino MJ, Anaya-Prado R. Incidence and predictive factors for cytomegalovirus infection in renal transplant recipients. Transplant Proc 2009; 41: 2412–5. [DOI] [PubMed] [Google Scholar]
- 21. Fernández-Ruiz M, López-Medrano F, Romo EM, et al. . Pretransplant lymphocyte count predicts the incidence of infection during the first two years after liver transplantation. Liver Transpl 2009; 15:1209–16. [DOI] [PubMed] [Google Scholar]
- 22. Nierenberg NE, Poutsiaka DD, Chow JK, et al. . Pretransplant lymphopenia is a novel prognostic factor in cytomegalovirus and noncytomegalovirus invasive infections after liver transplantation. Liver Transpl 2014; 20:1497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Calarota SA, Zelini P, De Silvestri A, et al. . Kinetics of T-lymphocyte subsets and posttransplant opportunistic infections in heart and kidney transplant recipients. Transplantation 2012; 93:112–9. [DOI] [PubMed] [Google Scholar]
- 24. Fernández-Ruiz M, López-Medrano F, Allende LM, et al. . Kinetics of peripheral blood lymphocyte subpopulations predicts the occurrence of opportunistic infection after kidney transplantation. Transpl Int 2014; 27:674–85. [DOI] [PubMed] [Google Scholar]
- 25. van den Berg AP, van Son WJ, Janssen RA, et al. . Recovery from cytomegalovirus infection is associated with activation of peripheral blood lymphocytes. J Infect Dis 1992; 166:1228–35. [DOI] [PubMed] [Google Scholar]
- 26. Gardiner BJ, Chow JK, Price LL, Nierenberg NE, Kent DM, Snydman DR. Role of secondary prophylaxis with valganciclovir in the prevention of recurrent cytomegalovirus disease in solid organ transplant recipients. Clin Infect Dis 2017; 65:2000–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ljungman P, Boeckh M, Hirsch HH, et al. ; Disease Definitions Working Group of the Cytomegalovirus Drug Development Forum Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis 2017; 64:87–91. [DOI] [PubMed] [Google Scholar]
- 28. Falagas ME, Snydman DR. Recurrent cytomegalovirus disease in solid-organ transplant recipients. Transplant Proc 1995; 27: 34–7. [PubMed] [Google Scholar]
- 29. Hanley PJ, Bollard CM. Controlling cytomegalovirus: helping the immune system take the lead. Viruses 2014; 6:2242–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shanahan A, Malani PN, Kaul DR. Relapsing cytomegalovirus infection in solid organ transplant recipients. Transpl Infect Dis 2009; 11:513–8. [DOI] [PubMed] [Google Scholar]
- 31. Humar A, Kumar D, Boivin G, Caliendo AM. Cytomegalovirus (CMV) virus load kinetics to predict recurrent disease in solid-organ transplant patients with CMV disease. J Infect Dis 2002; 186:829–33. [DOI] [PubMed] [Google Scholar]
- 32. Horton NJ, Kleinman KP. Much ado about nothing: a comparison of missing data methods and software to fit incomplete data regression models. Am Stat 2007; 61:79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Manuel O, Husain S, Kumar D, et al. . Assessment of cytomegalovirus-specific cell-mediated immunity for the prediction of cytomegalovirus disease in high-risk solid-organ transplant recipients: a multicenter cohort study. Clin Infect Dis 2013; 56:817–24. [DOI] [PubMed] [Google Scholar]
- 34. Giulieri S, Manuel O. QuantiFERON®-CMV assay for the assessment of cytomegalovirus cell-mediated immunity. Expert Rev Mol Diagn 2011; 11:17–25. [DOI] [PubMed] [Google Scholar]
- 35. Kumar D, Chernenko S, Moussa G, et al. . Cell-mediated immunity to predict cytomegalovirus disease in high-risk solid organ transplant recipients. Am J Transplant 2009; 9:1214–22. [DOI] [PubMed] [Google Scholar]
- 36. Kumar D, Mian M, Singer L, Humar A. An interventional study using cell-mediated immunity to personalize therapy for cytomegalovirus infection after transplantation. Am J Transplant 2017; 17:2468–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.