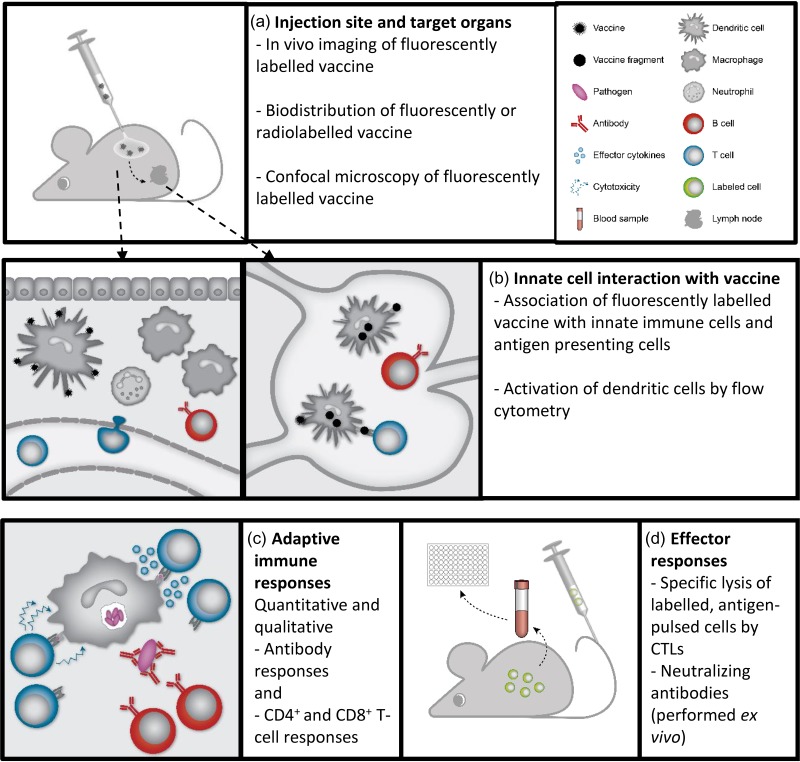
Figure 2.
Evaluation of vaccines—from identification of target cells to desired immune response profile. A number of different assays can be utilized to assess adjuvanted subunit vaccine function and efficacy. (A) Assays evaluating biodistribution and organ localization of vaccine components on a whole-body level are often based on detection of fluorescently or radiolabeled adjuvants and antigens. (B) In target organs such as the injection site and draining lymph nodes, association of vaccine components with innate immune cells and antigen-presenting cells can be evaluated using flow cytometry-based assays. These assays can be used to elucidate the mechanism of action for vaccine adjuvants and which antigen presenting cells that are activated by the adjuvant. (C) Qualitative and quantitative evaluation of the adaptive immune responses to subunit vaccines is used to assess vaccine efficacy. Quantitative responses can be measured by ELISA, ELISPOT, and immunoplex assays, whereas flow cytometry-based assays and antibody avidity assays can be used to evaluate the qualitative immune responses. (D) Vaccine efficacy can be assessed using alternative assays to pathogen challenge models. The cytotoxic potential of CD8+ T cells can be evaluated in antigen-specific lysis assays, while antibody functionality may be evaluated in neutralization assays or with methods to assess antibody Fc-dependent functionalities such as cytotoxicity, complement activation or phagocytosis.