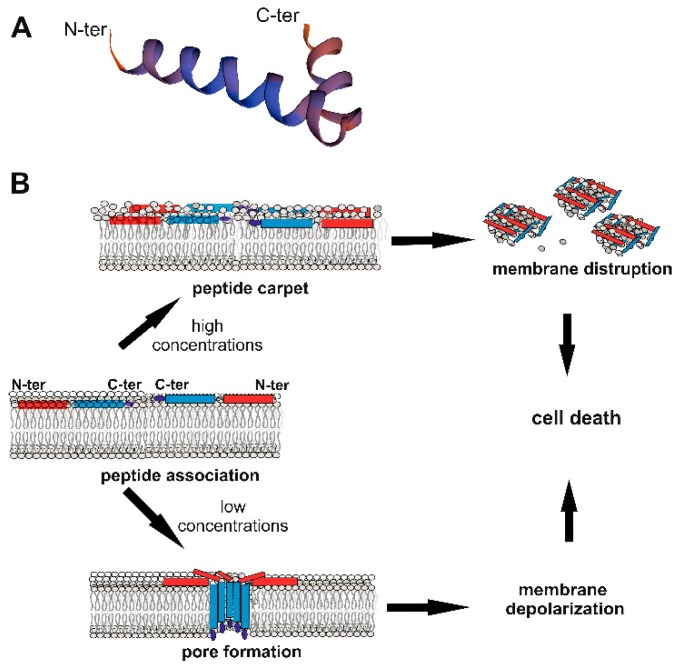
Figure 2.
Cecropin (Cec) structure and mechanisms of action against bacteria. (A) Structure of the mature 35 aa B. mori Q53 Cec B natural variant [53] obtained using SWISS-MODEL (Available online: https://swissmodel.expasy.org/), showing N- and C-terminal α-helices linked through a flexible hinge region. (B) Model of action against bacteria. Cecs associate with the bacterial membrane, with the long axes of the α−helical domains parallel to the lipid bilayer surface. Polar residues interact with the lipid phosphates; non-polar residues bury in the hydrophobic core of the membrane. At high concentrations (upper part), Cecs form a carpet-like structure with detergent-like properties, disrupting membranes. At lower concentrations (lower part), Cecs form pores, which affect the cellular electrolyte balance, causing bacterial death [85]. The pore is formed of different Cec molecules organized as oligomers, with C-terminal hydrophobic domains submerged into the phospholipidic hydrophobic chains [86]. The red rectangle represents the N-terminal helix, the blue one the C-terminal helix; the dark blue ellipse indicates the C-terminal amidated residue.