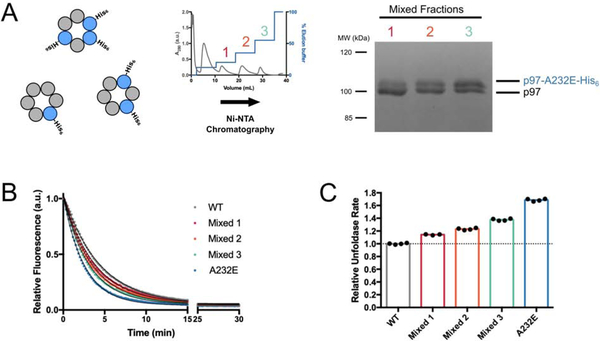
Figure 2. Unfoldase rates correlate with the number of mutant subunits in mixed p97 hexamers.
(A) Purification scheme for mixed hexamers. Wild-type and His6-tagged A232E-mutant p97 were co-expressed in E. coli, three fractions of mixed hexamers with increasing numbers of p97-A232E protomers were purified by stepwise elution from a Ni-NTA column (middle), and their relative composition was analyzed by SDS PAGE and Coomassie staining (right). (B) Example traces for the fluorescence decrease their single-exponential fits during single-turnover substrate unfolding by mixed p97 hexamers. (C) Relative rates for substrate unfolding by mixed hexamers and p97-A232E homo-hexamers compared to wild-type homo-hexamers. Technical replicates shown.