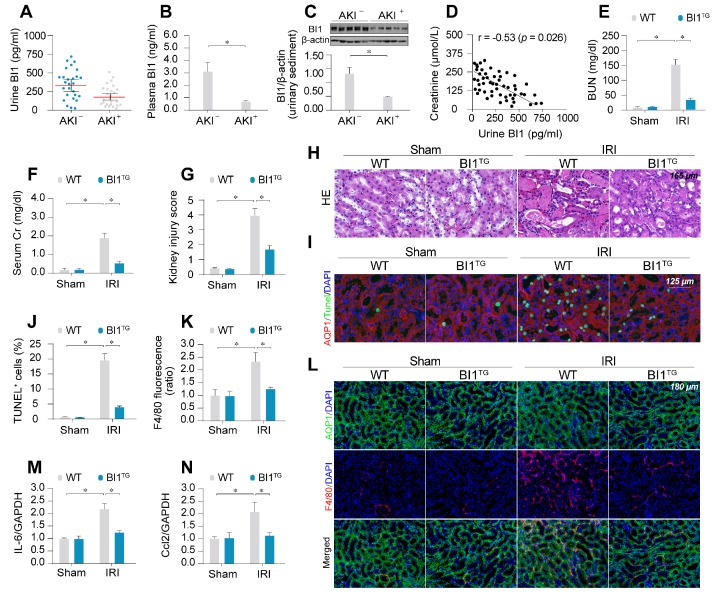
Figure 1.
BI1 level is suppressed in ischemic AKI. (A-B) BI1 protein levels in urine and plasma from AKI patients using ELISA. (C) Urine sediments were collected from patients with AKI or otherwise healthy controls and then western blotting was used to evaluate the expression of BI1 in urine. (D) Correlation between urine BI1 content and peak serum creatinine levels in patients with AKI. (E-F) Levels of BUN and serum creatinine from BI1 transgenic (BI1TG) and wild-type (WT) mice subjected to renal ischemia-reperfusion injury (IRI) in vivo. (G-H) Structural alteration of tubule after IRI using H&E staining. Semi-quantitative analysis of tubular injury (tubular atrophy or dilatation, loss of brush border, vacuolization, epithelial cell shedding, and denuded tubular basement membrane) was scored as: 0, normal; 1, <10%; 2, 10%-25%; 3, 25%-50%; 4, 50%-75%; 5, 75%-100% of affected area from 20 random fields. (I-J) Tubule death determined using TUNEL staining. AQP1 was employed to stain proximal tubule. (K-L) Immunofluorescence assay for F4/80 pro-inflammatory cells. (M-N) RNA was isolated from reperfused kidneys and transcriptions of Ccl2 and IL-6 were determined using qPCR. Experiments were repeated at least three times and data are shown as mean ± SEM (n = 6 mice or 3 independent cell isolations per group). *p<0.05.