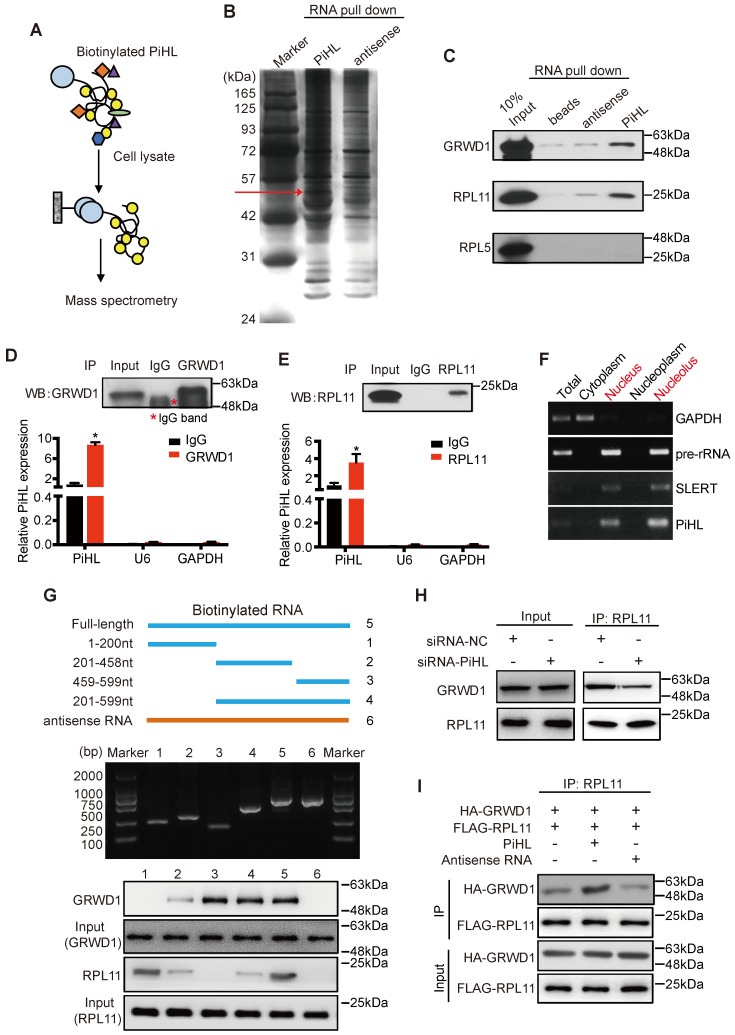
Figure 3.
PiHL interacts with GRWD1 and RPL11 complex. (A) experimental design for pull-down assays and identification of PiHL-associated cellular proteins. PiHL and antisense-PiHL RNA were biotinylated by in vitro transcription, refolded, and incubated with HCT116 total cell lysates. (B) Silver staining of biotinylated PiHL-associated proteins. One PiHL-specific band (red arrow) was excised and analyzed by mass spectrometry. (C) Western blot of GRWD1, RPL11 and RPL5 proteins retrieved by in-vitro-transcribed biotinylated PiHL from PiHL cell nuclear extracts. Antisense PiHL and beads were used as negative controls. (D, E) HCT116 lysates were immunoprecipitated with anti-GRWD1 antibodies (D), anti-RPL11 antibody (E), or control IgG. Aliquots of cell lysates (20% of input) and IgG, GRWD1 or RPL11 immunoprecipitates were separated by SDS-PAGE, and the specific immunoprecipitation of GRWD1 and RPL11 was confirmed by Western blot (WB) (upper). The complexes were analyzed for the presence of PiHL, U6 or GAPDH by qRT-PCR (lower). Signals were normalized to actin mRNA. Results are mean ± s.e.m of three independent experiments. (F) PiHL accumulates to the nucleolus. Total RNA from HCT116 cells was separated into cytoplasmic, nuclear, nucleoplasmic, and nucleolar fractions and analyzed by RT-PCR. GAPDH RNA serves as a positive control for cytoplasmic gene expression, GAPDH and SLERT as positive controls for nucleolus separation. (G) Western blot of GRWD1 and RPL11 in samples pulled down by truncated (∆1: 1-200, ∆2: 201-458, ∆3: 459-599, ∆4: 201-599), full-length (5) or antisense (6) PiHL. (H) immunoprecipitation assay was performed to detect the interaction between GRWD1 and RPL11 after transfection of PiHL siRNA. The 20% of input (cell lysate) and RPL11 immunoprecipitates were confirmed by Western blot. (I) PiHL promoted the binding between recombinant GRWD1 and RPL11 in vitro. Data shown represent three independent experiments.