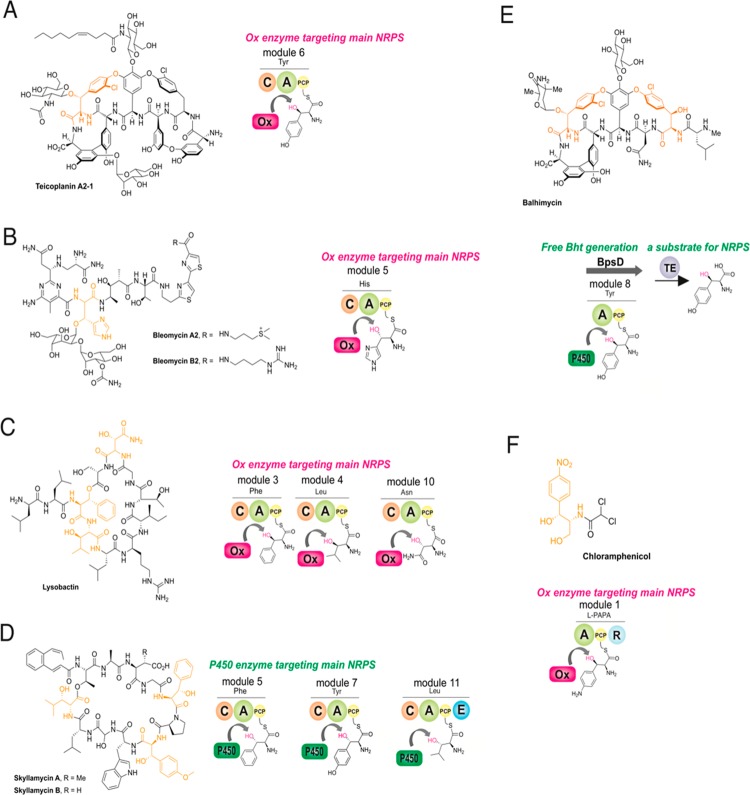
Figure 2.
Alternate routes for the incorporation of β-hydroxy amino acids in NRPS-mediated peptide biosynthesis. (A) Hydroxylation of the Tyr6 residue by a diiron monooxygenase in type IV GPA biosynthesis occurs during peptide assembly with the residue covalently attached to module 6 of the NRPS. (B,C) Modification of amino acids by diiron monooxygenases during peptide assembly in bleomycin (His8) and lysobactin (Phe3, Leu4, and Asn10) biosynthesis. (D) Multiple oxidation of different amino acids in the biosynthesis of skyllamycin (Phe5, OMe-Tyr7, Leu11) by a cytochrome P450 monooxygenase. (E) Provision of Bht precursors for the NRPS assembly lines that form type I–III GPAs utilizes a P450-mediated hydroxylation of Tyr on a separate, dedicated NRPS module. (F) Activity of the diiron monooxygenase CmlA toward PCP-bound 4-aminophenylalanine in chloramphenicol biosynthesis.