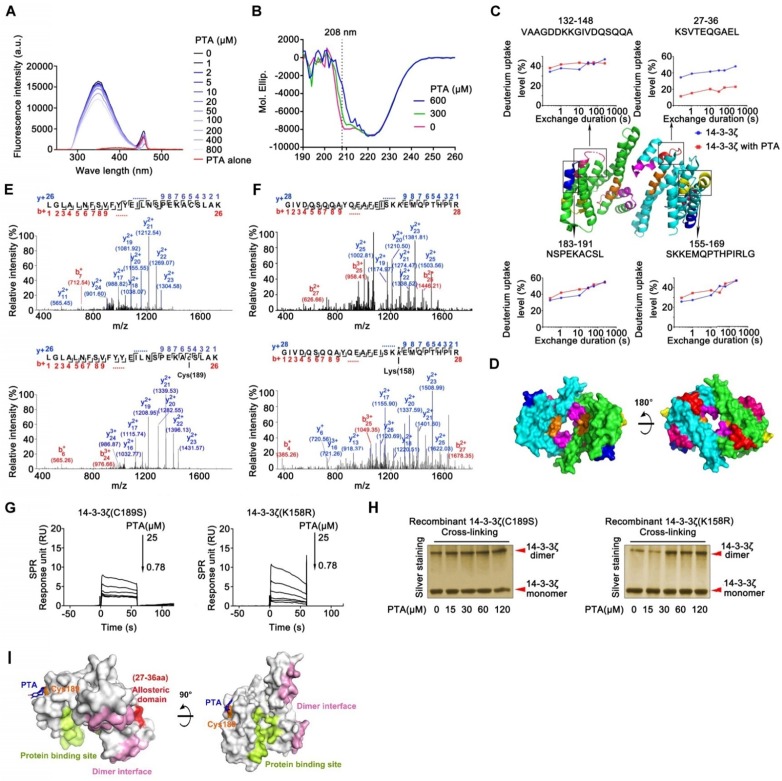
Figure 2.
Cysteine189 is a druggable allosteric site for 14-3-3ζ dimerization. (A) Fluorescence spectroscopy analysis of interaction of 14-3-3ζ with PTA. (B) CD spectra analysis for PTA-mediated 14-3-3ζ conformational change. (C) HDX profiles of 14-3-3ζ. Peptides with different HDX profiles in 14-3-3ζ are highlighted in different colors on X-ray crystal structure of 14-3-3ζ (PDB: 2C1N). Deuterium uptake plots of these peptides are presented. (D) Allosteric domains were shown in 3D structure of 14-3-3ζ dimer. (E) LC-MS/MS analysis of PTA-binding site on C189 of 14-3-3ζ. (F) LC-MS/MS analysis of PTA-binding site on K158 of 14-3-3ζ. (G) SPR analysis of PTA binding to 14-3-3ζ mutant (C189S) and SPR analysis of PTA binding to 14-3-3ζ mutant (K158R). (I) C189S but not K158R mutation suppressed PTA-dependent 14-3-3ζ dimerization. Silver-staining SDS PAGE analysis of PTA-dependent 14-3-3ζ mutant (C189S and K158R) dimerization. (K) Molecular docking of PTA binding to 14-3-3ζ.