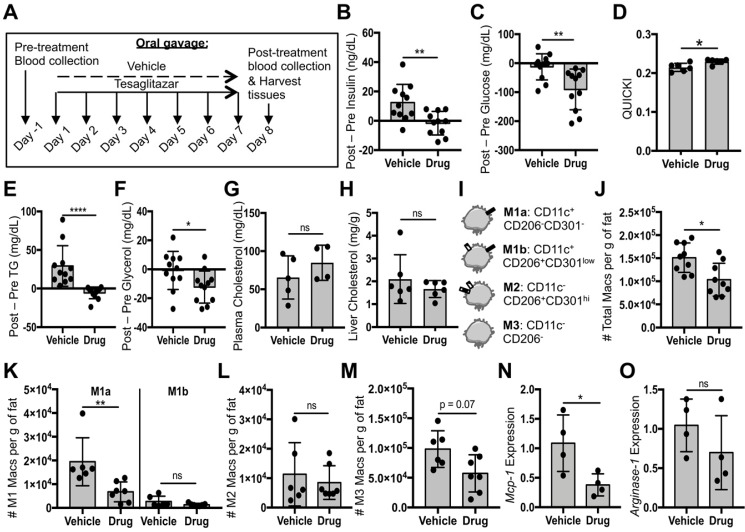
Figure 4.
Tesaglitazar delivered as a standard oral formulation improved metabolic parameters and reduced total macrophage and pro-inflammatory macrophage numbers in white adipose tissue. (A) Male ob/ob mice were treated daily by oral administration of tesaglitazar or vehicle for seven days. To assess metabolic effects, blood was harvested from mice before and after treatments and plasma was harvested. (B,C) Circulating insulin (B) and glucose (C) levels before and after treatment were measured and the changes in levels per mouse were calculated. (D) Post-treatment levels were also utilized to quantify QUICKI index for each mouse. (E-G) Circulating triglyceride (E), glycerol (F), and cholesterol (G) levels before and after treatment were measured and the changes in levels per mouse were calculated. (H) Post-treatment cholesterol levels in the liver were also quantified. (I) Epid SVF cells from ob/ob mice were stained with antibodies against markers of macrophages and macrophage subsets to quantify cell numbers by flow cytometry. (J) Total CD45+CD11b+F4/80+ macrophage numbers from epididymal adipose were normalized to the total mass of the adipose depot. (K-M) M1a and M1b (K), M2 (L), and M3 (M) macrophage subsets were quantified and normalized to total adipose mass as well. (N,O) RNA was extracted from sorted CD45+CD11b+F4/80+ peritoneal macrophages and macrophage chemokine Mcp-1 (N) and M2 marker Arginase-1 (O) expression levels were quantified. Data represents the mean ± SD; * p ≤ 0.05, ** p ≤ 0.01, **** p ≤ 0.0001. Vehicle indicates animals treated orally with vehicle, drug indicates animals treated orally with tesaglitazar.