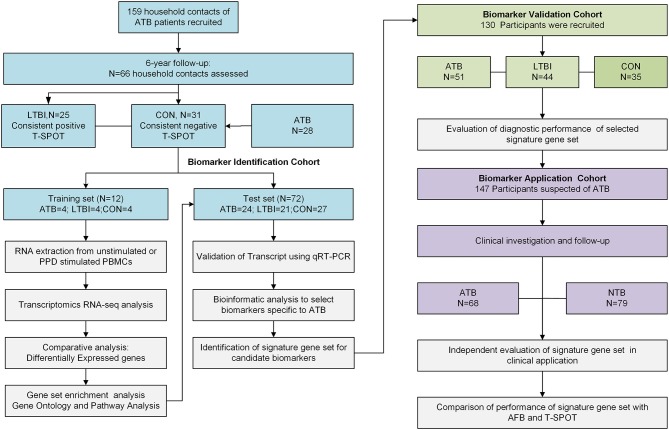
Figure 1.
Overall study design and subjects in the Biomarker Identification, Biomarker Validation and Biomarker Application cohorts. The subjects in LTBI and CON group from Biomarker Identification Cohort were recruited from household contacts of ATB patients from a prospective study during 6-year follow up. Subjects in this cohort were assigned to either the training set or the test set randomly. Subjects in training set were selected for RNA-seq. The differentially expressed genes found by RNA-seq were tested in the test set and then validated in an independent Biomarker Validation Cohort by qRT-PCR. The identified diagnostic gene signature was then applied in the clinical-based Biomarker Application Cohort. ATB group, active TB patients; LTBI group, subjects with latent tuberculosis infection; CON group, TB-uninfected controls; NTB group, patients without ATB; qRT-PCR, quantitative real-time PCR; T-SPOT, T-SPOT®. TB test (Oxford Immunotec Ltd, Oxford, UK); AFB, acid-fast bacilli.