Abstract
Idiopathic pulmonary fibrosis is a progressively fatal disease with limited treatments. The bleomycin mouse model is often used to simulate the disease process in laboratory studies. The aim of this study was to develop an ex vivo technique for assessing mice lung injury using lung ultrasound surface wave elastography (LUSWE) in the bleomycin mouse model. The surface wave speeds were measured at three frequencies of 100, 200, and 300 Hz for mice lungs from control, mild, and severe groups. The results showed significant differences in the lung surface wave speeds, pulse oximetry, and compliance between control mice and mice with severe pulmonary fibrosis. LUSWE is an evolving technique for evaluating lung stiffness and may be useful for assessing pulmonary fibrosis in the bleomycin mouse model.
Keywords: Idiopathic pulmonary fibrosis (IPF), Lung ultrasound surface wave elastography (LUSWE), Mice, Bleomycin (BLM), Surface wave speed
Introduction
Idiopathic pulmonary fibrosis (IPF) is a progressive fibrotic lung disease with limited available treatment options (Tashiro et al., 2017). In aging tissues, collagen fibers may be prone to inappropriate cross-linking leading to tissue stiffening and decreased elasticity (Robins, 2007). Currently, the bleomycin (BLM) model of lung fibrosis is the most extensively used laboratory animal model for pulmonary fibrosis, though the model continues to have many drawbacks including variable reproducibility. In this animal model, lung injury is induced by BLM and the subsequent healing response is manifested as increased collagen deposition, patchy fibrosis, and inflammatory infiltrates. There are few current standards for classifying fibrotic lung disease in animal studies.
Ultrasonography is not widely used in the clinic for assessing lung disease because ultrasound energy cannot penetrate deep into the lung parenchyma. We developed a lung ultrasound surface wave elastography (LUSWE) technique to noninvasively measure the surface wave speed of lung for assessing superficial lung disease (Clay et al., 2018; Zhang et al., 2017). LUSWE is useful for assessing patients with interstitial lung disease (ILD) because they have fibrotic lung injury mostly distributed in the peripheral, subpleural regions of the lungs (Zhang et al., 2017; Zhou et al.). The purpose of this research was to develop an ex vivo technique for quantifying mice lung injury due to bleomycin. The surface wave speeds were analyzed for ex vivo healthy, mild, and severe mice lungs.
Materials and methods
Female C57 black mice (Jackson Laboratories, Bar Harbor, ME, USA) were used for experiments and handled according to the standard care and methodologies approved by the Mayo Clinic Institutional Animal Care and Use Committee. Eight-week-old (~20 g) mice were administered bleomycin (BLM) (Millipore Sigma, CAS 9041-93-4) (0.04–0.06 U diluted in 50 μl of 0.9% normal saline) or 50 μl of 0.9% normal saline alone by tracheal instillation using an intratracheal aerosolizer (Penn-Century, Wyndmoor, PA, USA) on day 0 while under ketamine/xylazine anesthesia. While still anesthetized, mice were shaved around the collar region to allow monitoring of dissolved oxygen levels on room air using a MouseOx collar clip monitoring system (Starr Life Sciences Corp., Holliston, MA, USA) and ear tagged for identification.
Mouse weight, heart rate, respiratory rate, and dissolved oxygen, as well as, overall well-being were monitored twice a week for 3 weeks. This refers to the Pulse Oximetry measurement which was done on days 4, 7, 11, 14 and 21 post bleomycin instillation. Prior to sacrifice, BLM-challenged mice were categorized into 2 groups (mild and severe). Mice were evaluated based on the monitored indicators of health, and each treatment group was populated with 8 animals along with a control group of 6 mice. On day 21, mice were euthanized by overdose of pentobarbital (100 mg/kg). Immediately following euthanasia, the compliance of the respiratory system was measured using the flexiVent system (SCIREQ, Montreal, Canada) running the program for PVr-P perturbation. Subsequently, lungs were dissected and samples were collected for the ultrasound procedure described below.
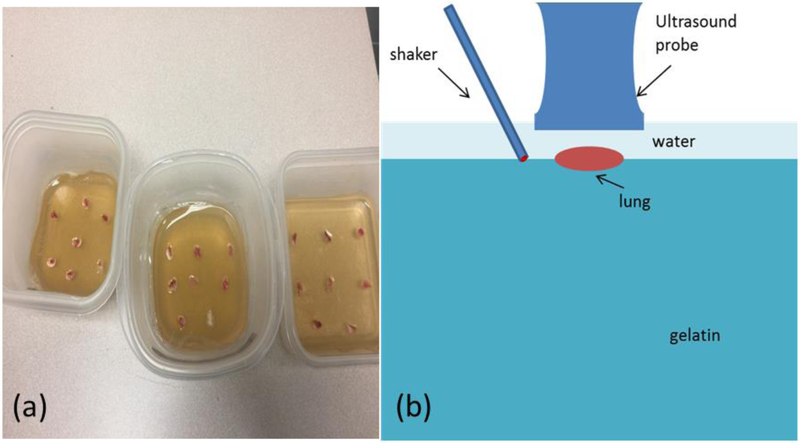
The mouse lungs were immersed in saline and transported to the ultrasound laboratory for experimental preparation. A gelatin mixture was prepared from porcine skin gelatin (SIGMA-ALDRICH, Inc). The gelatin mixture was heated to 60°C and then cooled to room temperature. The cooled gelatin mixture was poured into three plastic containers labelled as control, mild, and severe lung injury groups. The lungs from the control, mild, and severe groups were placed onto the gelatin in the corresponding containers. The lungs were on top of the gelatin as shown in Figure 1a. The lungs and gelatin stayed overnight in room temperature to make sure they were completely bonded together. Given the size of the mice lung is small, it is difficult to generate the wave propagation directly on the lung. The gelatin phantom was used for wave generation and supporting the small lung sample for wave propagation through the small sample. This method provides a mean for testing other small samples. The gelatin can also decay wave energy and reduce wave reflection from the boundaries of the testing container.
Figure 1.
(a) Three groups of mouse lungs from control, mild, and severe were prepared on gelatin in plastic containers for measuring the surface wave speed of lung; (b) schematic of experimental setup.
To improve the experiment, ultrasound transmission gel was not used; rather water was used for imaging the lung. A high frequency linear array ultrasound probe L22–14LF with central frequency of 18 MHz (Verasonics, Inc, Kirkland, WA) was positioned over the lung. An electromagnetic shaker with a spherical indenter (4 mm in diameter) was positioned adjacent to the ultrasound probe. A harmonic vibration at 100, 200, and 300 Hz with a duration of 0.1 second was generated by a function generator (Model: FG33120A, Hewlett Packard, Palo Alto, CA), amplified by an audio amplifier, and transmitted through the indenter of the shaker on a location on the gelation surface (Fig. 1b) (Zhou and Zhang, 2019). A Verasonics ultrasound system (Vantage 1, Verasonics; Kirkland, WA) was used to collect two thousand imaging frames per second using a plane-wave pulse transmission method (Zhang et al., 2018b; Zhang et al., 2019). Measurements were performed 3 times at each condition.
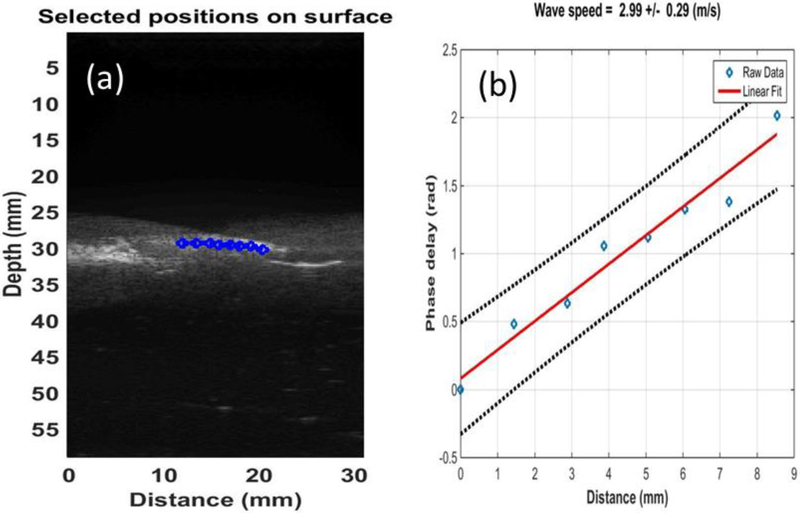
The tissue motion was estimated using autocorrelation technique for displacement tracking (Zhou et al., 2017). The wave speed of lung surface was calculated based on the phase-delay of selected points as a function of distance. The phase delay was calculated using the cross-correlation technique. The wave speed was measured by ultrasound probe and estimated using a phase gradient method
| (1) |
where ν is the wave speed to be determined, f is the excitation frequency in Hz and α is the slope of the linear regression between phase delay and lateral distance (Clay et al., 2018; Zhang et al., 2018a; Zhou and Zhang, 2018) (Fig. 2).
Figure 2.
(a) 8 locations over a length of approximately 8 mm on the lung surface were used to measure the lung surface wave speed using ultrasound tracking beams; (b) the wave phase delay of the remaining locations, relative to the first location, is used to measure the surface wave speed.
Statistical analysis
A one-way ANOVA test was performed to compare the sample means of wave speed at 3 frequencies; pulse oximetry; and compliance among control, mild, and severe lung injury groups. Multiple comparisons were then performed. Differences in mean values were considered significant when p <0.05.
Results
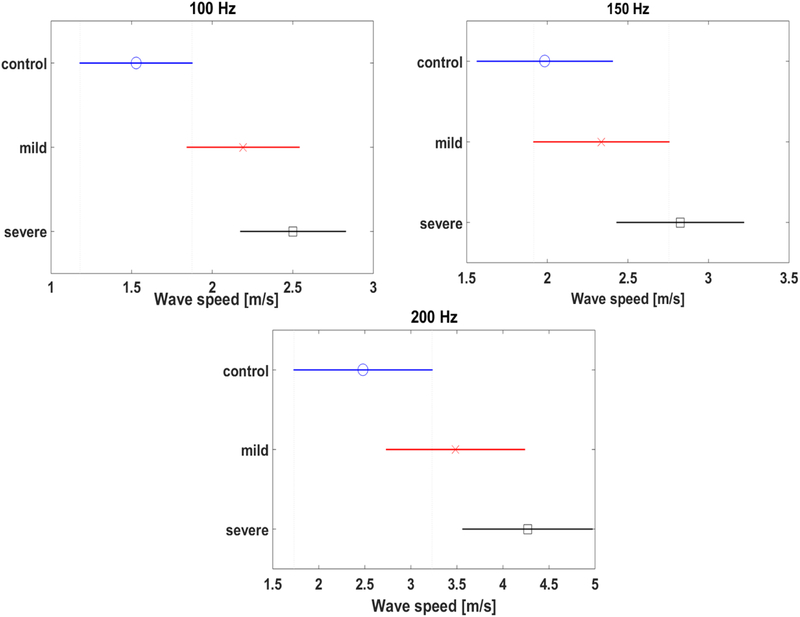
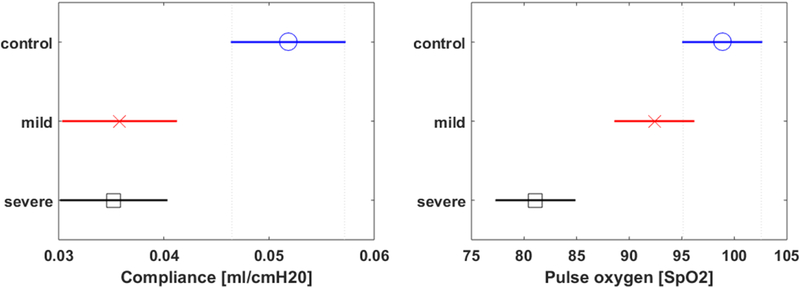
23 individual mouse lungs (control: 7, mild: 8, severe: 8) were evaluated in this study. A comparison of lung surface wave speeds of mice among control, mild, and severe pulmonary fibrosis groups is shown in Figure 3 for 100, 200, and 300 Hz. There were statistically significant differences in the wave speeds at the three frequencies between the control and severe groups. A comparison of pulse oximetry and compliance among control, mild, and severe groups is shown in Figure 4. There were statistically significant differences in the pulse oximetry and compliance between control and severe groups. Given the small number of mice were analyzed in the three groups, the statistical power for separating the three groups were not high. This was demonstrated in Figure 3 as shown relatively overlaps of wave speed among the three groups. There were relatively overlaps in pulse oximetry and compliance among the three groups as shown in Figure 4. We will study the statistical power for separating the three groups with relatively large samples in future.
Figure 3.
Comparison of wave speeds among control, mild, and severe groups. Surface wave speed at (a) 100 Hz, (b) 150 Hz, (c) 200 Hz.
Figure 4.
Comparison of pulse oximetry (a) and compliance (b) among control, mild, and severe groups.
Discussion
The aim of this study was to develop an ex vivo technique for assessing mouse lung injury in the bleomycin mouse model. Because the mouse lung is small, it is difficult to apply a direct force on the lung. We put the mouse lung on the gelatin. Wave propagation can be generated on the gelatin and travel through the lung. Therefore, surface wave propagation on the lung can be measured. This technique can also be applied to other small tissues.
Significant differences in wave speeds at three excitation frequencies, pulse oximetry, and compliance of the mouse lungs were observed between the control and severe injury groups, yet these measurements were not statistically different between mild and severe groups. The results obtained in this study are within the range of published results for other species. The surface wave speed of lung was found to be 1.53 ± 0.48 m/s for healthy controls at 100 Hz, which is similar to values reported for porcine lungs (1.83 m/s at 100 Hz) (Zhang, 2011). Experimental results showed that the wave speed of mouse lung surface of different groups increased with vibration frequency, which is commonly observed (Zhang et al., 2008). Moreover, significant differences in wave speed between control and severe groups were observed at three frequencies. This is consistent with the findings in our clinical study in interstitial lung disease (Zhang et al., 2017). Meanwhile, significant differences in the compliance and pulse oximetry between the controls and severe groups were observed. This raises the potential of assessing lung stiffness using LUSWE for longitudinally tracking and monitoring progression of disease or response to therapy in research studies.
The lung tissue may be considered as linear in LUSWE because the generated wave motion is very small and typically less than 10 um. The reason we use 3 different frequencies to generate wave and measure the resulted wave speed is that we want to use Voigt’s model to characterize the viscoelasticity of lung tissue. However, it is difficult to do this since the lung mass density in vivo is not available to obtain noninvasively. We are currently developing a deep neural network technique to predict the lung mass density based on the wave speeds at different excitation frequencies as well as measurements from pulmonary function test. Moreover, lung tissue is often regarded as porous material. We plan to quantify the porous viscoelasticity of lung tissue using both LUSWE and deep neural network technique in future.
It is likely the lung pathology/injury is non-uniform. The surface wave measurement in LUSWE is local and only dependent on the local material property. For human studies, the measurements of surface wave speed can be obtained from multiple local regions and averaged to get a global measurement. In our previous study, we found that global changes in lung surface wave speed correlated with clinical assessment of disease progression. Local measurements at each intercostal space may provide sensitive information on disease progression.
Since the mice lung is small, the surface wave speed is measured over the long axis of the lung. One of main purposes is to measure the wave speed in small tissue samples such as the ex vivo lung in this research. We will investigate the heterogeneous property of small tissue samples using LUSWE in future studies.
The bleomycin mouse model of fibrosis has yielded inconsistent results when used to predict effective treatments for humans with IPF (Moeller et al., 2008). The model itself is not completely analogous to IPF and accurate noninvasive detection of fibrosis has been elusive. Previous attempts to improve the longitudinal detection of incipient and progressing pulmonary fibrosis have focused around MRI technology (Caravan et al., 2013; Karmouty - Quintana et al., 2007; Velde et al., 2014). While the developed techniques have proof of accuracy, they still add a layer of complexity that is not easily translated to large scale animal studies that are needed for accurate drug development. The LUSWE ultrasound imaging technique is a more accessible technique for large scale animal studies and could potentially provide an objective measure of fibrosis for laboratory based studies of lung fibrosis and treatment strategies. There is need for additional experimental investigations of LUSWE for assessing lung stiffness for pulmonary fibrosis in the BLM mouse model, especially in vivo.
Conclusion
An ex vivo technique is developed for assessing mice lung injury in the bleomycin mouse model. The data are presented as wave speed (at 100, 200, and 300 Hz), pulse oximetry, and compliance of mouse lungs among three groups. The results show significant differences in wave speed, compliance, and pulse oximetry between control and severe mouse groups. Results from this study suggest that lung surface wave speed may be used to assess the fibrotic response of bleomycin in the mouse model to aid in research studies.
Acknowledgements
This study is supported by NIH R01HL125234 from the National Heart, Lung, and Blood Institute. We thank Mrs. Jennifer Poston for editing this manuscript.
Footnotes
Conflict of Interest Statement
There are no conflicts of interest.
References
- Caravan P, Yang Y, Zachariah R, Schmitt A, Mino-Kenudson M, Chen HH, Sosnovik DE, Dai G, Fuchs BC, Lanuti M, 2013. Molecular magnetic resonance imaging of pulmonary fibrosis in mice. American journal of respiratory cell and molecular biology 49, 1120–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay R, Bartholmai BJ, Zhou B, Karwoski R, Peikert T, Osborn T, Rajagopalan S, Kalra S, Zhang X, 2018. Assessment of Interstitial Lung Disease Using Lung Ultrasound Surface Wave Elastography: A Novel Technique With Clinicoradiologic Correlates. J Thorac Imaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmouty - Quintana H, Cannet C, Zurbruegg S, Blé FX, Fozard JR, Page CP, Beckmann N, 2007. Bleomycin - induced lung injury assessed noninvasively and in spontaneously breathing rats by proton MRI. Journal of Magnetic Resonance Imaging 26, 941–949. [DOI] [PubMed] [Google Scholar]
- Moeller A, Ask K, Warburton D, Gauldie J, Kolb M, 2008. The bleomycin animal model: a useful tool to investigate treatment options for idiopathic pulmonary fibrosis? The international journal of biochemistry & cell biology 40, 362–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins S, 2007. Biochemistry and functional significance of collagen cross-linking. Portland Press Limited. [DOI] [PubMed] [Google Scholar]
- Tashiro J, Rubio GA, Limper AH, Williams K, Elliot SJ, Ninou I, Aidinis V, Tzouvelekis A, Glassberg MK, 2017. Exploring animal models that resemble idiopathic pulmonary fibrosis. Frontiers in medicine 4, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velde GV, De Langhe E, Poelmans J, Dresselaers T, Lories RJ, Himmelreich U, 2014. Magnetic Resonance Imaging for Noninvasive Assessment of Lung Fibrosis Onset and Progression: Cross-Validation and Comparison of Different Magnetic Resonance Imaging Protocols With Micro–Computed Tomography and Histology in the Bleomycin-Induced Mouse Model. Investigative radiology 49, 691–698. [DOI] [PubMed] [Google Scholar]
- Zhang X, Kinnick R, Greenleaf JF, Year Viscoelasticity of lung tissue with surface wave method. In 2008. IEEE Ultrasonics Symposium. [Google Scholar]
- Zhang X, Osborn T, Zhou B, Meixner D, Kinnick R, Bartholmai B, Greenleaf J, Kalra S, 2017. Lung ultrasound surface wave elastography: a pilot clinical study. IEEE transactions on ultrasonics, ferroelectrics, and frequency control 64, 1298–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Qiang B, Hubmayr RD, Urban MW, Kinnick R, Greenleaf JF, 2011. Noninvasive ultrasound image guided surface wave method for measuring the wave speed and estimating the elasticity of lungs: A feasibility study. Ultrasonics 51, 289–295. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhou B, Miranda AF, Trost LW, 2018a. A Novel Noninvasive Ultrasound Vibro-elastography Technique for Assessing Patients With Erectile Dysfunction and Peyronie Disease. Urology 116, 99–105. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhou B, Osborn T, Bartholmai B, Kalra S, 2018b. Lung ultrasound surface wave elastography for assessing interstitial lung disease. IEEE Transactions on Biomedical Engineering, 1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhou B, VanBuren WM, Burnett TL, Knudsen JM, 2019. Transvaginal Ultrasound Vibro-elastography for Measuring Uterine Viscoelasticity: A Phantom Study. Ultrasound in Medicine & Biology 45, 617–622. [DOI] [PubMed] [Google Scholar]
- Zhou B, Bartholmai BJ, Kalra S, Osborn TG, Zhang X, Lung US Surface Wave Elastography in Interstitial Lung Disease Staging. Radiology 0, 181729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Sit AJ, Zhang X, 2017. Noninvasive measurement of wave speed of porcine cornea in ex vivo porcine eyes for various intraocular pressures. Ultrasonics 81, 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Zhang X, 2018. Comparison of five viscoelastic models for estimating viscoelastic parameters using ultrasound shear wave elastography. Journal of the Mechanical Behavior of Biomedical Materials 85, 109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Zhang X, 2019. The effect of pleural fluid layers on lung surface wave speed measurement: Experimental and numerical studies on a sponge lung phantom. Journal of the Mechanical Behavior of Biomedical Materials 89, 13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]