Abstract
Locus coeruleus (LC) tau accumulation begins early. Targeting LC (dys)function might improve early identification for Alzheimer’s disease (AD) risk. Pupillary responses during cognitive tasks are driven by the LC and index cognitive effort. Despite equivalent task performance, adults with mild cognitive impairment (MCI) have greater pupil dilation/effort during digit span than cognitively normal (CN) individuals. We hypothesized that AD polygenic risk scores (AD-PRSs) would be associated with pupillary responses in middle-aged CN adults. Pupillary responses during digit span tasks were heritable (h2=.30–.36) in 1119 men ages 56–66. In a CN subset—all with comparable span capacities (n=539)—higher AD-PRSs were associated with greater pupil dilation/effort in a high (9-digit) cognitive load condition (Cohen’s d=.36 for upper versus lower quartile of AD-PRS distribution). Results held up after controlling for APOE genotype. Results support pupillary response—and by inference, LC dysfunction—as a genetically-mediated biomarker of early MCI/AD risk. In combination with other biomarkers, task-evoked pupillary responses may provide additional information for early screening of genetically at-risk individuals even before cognitive declines.
Keywords: polygenic risk score, risk indicator, biomarker, early identification, locus coeuruleus, mild cognitive impairment
1. Introduction
Alzheimer’s disease (AD) is a worldwide public health problem and the most costly disease in the United States (Alzheimer’s Association, 2018). Pathological changes begin decades before onset of dementia, making early identification of AD risk of paramount importance for slowing disease progression (Golde et al., 2011; Sperling et al., 2011). Toward that end, we suggest that a focus on tau or tau-associated processes may be quite useful.
Postmortem data indicate that tau pathology is the earliest occurring AD biomarker, first appearing in the locus coeruleus (Braak and Del Tredici, 2011, 2012; Duyckaerts et al., 2015; Ehrenberg et al., 2017). Thus, focusing on tau or tau-associated processes may be quite useful. There is also CSF-based evidence indicating that tau pathology can precede Aß in people who progress to AD (de Leon et al., 2018). Tau is more strongly associated with cognition than Aß (Maass et al., 2018), and lower LC neuronal density has been associated with faster cognitive decline in cognitively normal (CN) adults, and individuals with mild cognitive impairment (MCI) and autopsy-confirmed AD (Wilson et al., 2013). Finally, an animal model has now suggested the plausibility of Braak’s model of the AD pathological process beginning in the LC by showing pretangle spread via abnormal LC tau production and progression associated with cognitive decline, with these changes occurring in the absence of amyloid (Ghosh et al., 2019).
There are established positron emission tomography and cerebrospinal fluid (CSF) beta-amyloid (Aß) and tau biomarkers, but both are costly and invasive and the tau assays do not adequately detect tau in the LC. Thus, there is good reason to search for additional, noninvasive markers of risk that could serve as indicators of early LC function. Our prior work suggests that pupillary dilation during performance of cognitive tasks—which has been linked to LC function—is one such early marker of AD risk (Granholm et al., 2017). Here we sought to determine if this pupillary response is also a genetically-mediated biomarker. An abnormal light reflex—another pupillary response—has been linked to AD and Parkinson’s disease (Fotiou et al., 2009; Fotiou et al., 2000; Prettyman et al., 1997), but our focus here is on pupil dilation associated with cognitive effort rather than reflexive pupil constriction.
Increased pupillary dilation during performance of cognitive tasks is a validated, objective psychophysiological index of the brain’s cognitive resource allocation, i.e., cognitive effort (Ahern and Beatty, 1979; Beatty, 1982; Granholm et al., 1996; Kahneman and Beatty, 1966; van der Meer et al., 2010). Ability level is inversely related to amount of effort—indexed by amount of pupil dilation—needed to perform a task. Pupil size increases with increasing cognitive effort as task demand, i.e., cognitive load, increases (Ahern and Beatty, 1979; Beatty, 1982; Granholm et al., 1996; Granholm et al., 2017; Kahneman and Beatty, 1966; van der Meer et al., 2010). However, when task demands substantially exceed abilities and compensatory capacity, there is disengagement from the cognitive processing system; at that point, dilation drops off and performance declines (Ahern and Beatty, 1979; Beatty, 1982; Granholm et al., 1996; Granholm et al., 2017; van der Meer et al., 2010). These pupillary responses reflect activation in the LC (Alnaes et al., 2014; Aston-Jones and Cohen, 2005; Gilzenrat et al., 2010; Joshi et al., 2016; Koss, 1986; Murphy et al., 2014; Phillips et al., 2000; Raizada and Poldrack, 2007; Samuels and Szabadi, 2008). Although the LC has been viewed historically as important only in terms of broad arousal responses, Aston-Jones and Cohen’s (2005) adaptive gain model supports a complex role of the LC-noradrenergic (LC-NE) system involving phasic activation with adaptive gain to optimize task performance and tonic activation associated with gain to optimize appropriate disengagement and a shift of focus to different stimuli or tasks. Thus, the LC-NE system is an important modulator of cognitive function and management of cognitive load (Aston-Jones and Cohen, 2005; Coull et al., 1999; Samuels and Szabadi, 2008; Sara, 2009; Wilhelm et al., 1999).
Several lines of research are consistent with the modulatory role of the LC-NE system with respect to cognition. There is evidence of age differences in LC function (Lee et al., 2018) and structural MRI measures of the LC have been associated with cognitive function in older adults (Clewett et al., 2016; Hämmerer et al., 2018). We showed associations between fMRI resting-state BOLD variance and pupillary dilation responses in cognitively normal and MCI participants in a subset of those in the current study (Elman et al., 2017). Other evidence is consistent with activation of the LC-NE system playing a role in promoting cognitive reserve in older adults (Clewett et al., 2016; Mather and Harley, 2016).
In previous work, based on the modulatory role of the LC-NE system, we hypothesized that if two individuals had the same cognitive test score, the one needing more effort is at higher risk for decline because they would be closer to their maximum capacity for compensation (cf. Riediger et al., 2006; Stern et al., 2018). On the other hand, someone who has already experienced substantial declines and has surpassed their compensatory threshold is likely to have both poor performance and reduced pupillary dilation responses. Pupillary dilation responses should thus be most useful as a very early marker of risk while there is still little or no observable cognitive decline. Our prior work with participants in the present sample supports these ideas (Granholm et al., 2017). Individuals with single-domain amnestic MCI had elevated pupillary dilation responses at low or moderate processing loads during digit span tasks, despite equivalent performance to CN participants. Those with multiple-domain MCI—having more depleted resources—had both impaired performance and reduced pupillary responses. Similarly, patients with spatial neglect have very depleted attentional resources and also have reduced pupillary dilation responses during an attentional task (Walle et al., 2019).
Previously, we also showed that an AD polygenic risk score, which had been validated against both living and autopsy-confirmed AD cases (AD-PRS; Escott-Price et al., 2017a; Escott-Price et al., 2017b; Escott-Price et al., 2015), was associated with increased odds of MCI in participants from the present sample, 89% of whom were <60 years old (Logue et al., 2018). The odds ratio for MCI was 3.2 for the upper versus the lower quartile of the PRS distribution. Results changed little after accounting for the effects of APOE (Logue et al., 2018), the largest single genetic determinant of AD risk (Corder et al., 1993; Lambert et al., 2013). If the pupillary dilation responses were also associated with the AD-PRS, it would provide proof of concept supporting the validity and potential utility of pupillary dilation responses.
The literature supports the idea that pupillary dilation responses are associated with cognitive resource allocation and are linked to LC structure and function. LC tau deposition has also been shown to be present in early adulthood, and the modulatory role of the LC-NE system appears to be less efficient in older adults. Pupillary responses during digit span can differentiate cognitively normal midlife adults from those with amnestic MCI even before there are observable task performance differences. Higher AD-PRSs have also been associated with increased odds of having MCI. Taken together, these findings suggest that pupillary dilation responses themselves—and by inference, LC dysfunction—in mid- and later life may be AD-related.
Here we hypothesized that pupillary dilation responses during a cognitive task are a genetically-mediated AD risk indicator in late middle-aged adults. We used the classical twin design to estimate the heritability of pupillary responses, thereby demonstrating that they are genetically influenced (Eaves et al., 1978; Neale and Cardon, 1992). Next we tested our primary hypothesis that a higher AD-PRS would be associated with greater pupil dilation during a cognitive task even in cognitively normal middle-aged individuals. Having previously shown that pupillary dilation responses can differentiate cognitively normal midlife adults from those with MCI (Granholm et al., 2017), their association with the AD-PRS would contribute additional proof of concept supporting the validity of pupillary dilation responses as an early and genetically-mediated marker of AD-related risk.
2. Materials and methods
2.1. Participants
Participants were men in wave 2 of the Vietnam Era Twin Study of Aging (VETSA), a national, community-dwelling sample similar to American men in their age range with respect to health and lifestyle characteristics based on Center for Disease Control and Prevention data (Kremen et al., 2013; Schoeneborn and Heyman, 2009). All served in the military sometime between 1965 and 1975, but ~80% reported no combat exposure. The flow of participant selection is shown in Figure 1. Sample characteristics are shown Table 1 for twin/heritability analyses of all participants with pupillometry data (n=1119) and those with maximum digit spans of 5–7 for the AD-PRS analyses (n=539) (explained below). The average general cognitive ability percentile scores of 61.7 and 63.3 correspond to IQ of 104–105 (Lyons et al., 2017; Lyons et al., 2009). The threshold of 16 on the Center for Epidemiologic Studies Depression Scale (Radloff, 1977) was used to estimate the number clinical depression. Participants were also asked if they ever had a head injury with loss of consciousness or confusion; almost all were defined as mild and occurred an average of 35 years earlier (Kaup et al., 2019). Participants traveled to the University of California, San Diego or Boston University where identical protocols were implemented. Written informed consent was obtained from all participants, and the study was approved by Institutional Review Boards at participating institutions.
Fig. 1.
Flow chart of participant selection. EUR, participants of European-American ancestry.
Table 1.
Participant characteristics
| Full sample for twin analyses (N=1119) | Subsample for AD-PRS analyses (N=539) | |||||
|---|---|---|---|---|---|---|
| Characteristic | Mean/N | SD/% | Range | Mean/N | SD/% | Range |
| Age (y) | 61.7 | 2.4 | 56.0–66.9 | 61.7 | 2.4 | 56.5–66.9 |
| Education (y) | 13.8 | 2.1 | 5–50 | 13.8 | 2.1 | 6–20 |
| GCA | 61.7 | 21.8 | 2–99 | 63.3 | 20.7 | 9–99 |
| CES-D (# above clinical depression threshold (≥16) | 149 | 13.4% | ----- | 62 | 11.5% | ----- |
| History of head injury with loss of consciousness or confusion | 312 | 27.9% | ----- | 147 | 27.3% | ----- |
| APOE-ε4 carriers | 325 | 29.7% | ----- | 155 | 29.4% | ----- |
Key: GCA, general cognitive ability percentile score; CES-D, Center for Epidemiologic Studies Depression Scale (Radloff, 1977). Mean, standard deviation (SD), and range are presented for age, education and GCA. N and percent are presented for CES-D and history of head injury. Subsample for AD-PRS analyses were cognitively normal individuals with maximum digit span=5–7. Ns vary slightly for some measures due to missing data; percentages shown are the percent of participants with non-missing data.
The present study began with 1207 participants (Kremen et al., 2013; Kremen et al., 2006). Exclusions were: self-reported history of glaucoma in either eye, penetrating eye wounds to both eyes, surgery to both eyes involving the muscle, or use of cholinesterase inhibitors or prescribed ocular medications (n=57); or equipment failures or excessive blinking (n=34). Depression and head injury were not exclusions because they are risk factors for dementia. This left 1119 individuals with valid pupillometry data (Granholm et al., 2017) and 1085 with genotyping data who were of European ancestry. There were too few individuals of non-European ancestry to include in the AD-PRS analyses. There were 828 individuals who were both CN and had valid pupillometry data. Because we were primarily interested in examining whether pupil dilation can inform risk for AD when performance is comparable among individuals, we selected 539 of these 828 individuals with relatively similar maximum span capacities of 5–7 digits (see Discussion for further examination of this issue). Since our digit span task included 3-, 6-, and 9-digit conditions, max span for this subgroup was thus only ±1 digit from the moderate 6-digit load. These included 87 monozygotic (MZ) twin pairs, 62 dizygotic (DZ) twin pairs, and 241 unpaired twins.
2.2. Cognition
2.2.1. Definition of cognitively normal status
As described in detail elsewhere (Granholm et al., 2017; Kremen et al., 2014; Logue et al., 2018), cognitive status was determined on the basis of 18 neuropsychological tests covering 6 cognitive domains. Using the Jak-Bondi approach (Jak et al., 2009), MCI was defined as having ≥2 tests in a domain that were each >1.5 SDs below normative means. To ensure that MCI reflected a decline in function rather than lifelong low ability, these values were determined after adjusting for general cognitive ability which was assessed at an average age of 20 years (Lyons et al., 2017; Lyons et al., 2009). Individuals with no impaired domains (85%) were considered CN.
2.2.2. Digit span capacity
We used digit span tasks during pupillometry (see section 2.3). Maximum span capacity was defined as the longest string of digits correctly recalled during standard testing with the Wechsler Memory Scale-III digit span subtest without the pupillometer (Wechsler, 1997).
2.3. Pupillometry
We used handheld NeurOptics PLR-2000 pupillometers to record pupil diameter from one eye at 30 Hz for up to 15 seconds while participants viewed a set of lights around a dark interior (~200 lux) inside in a viewing tube. The pupillometer contains recording optics and has a 1.5-inch viewing tube that surrounds the eye and blocks ambient light. To block the other eye, participants closed and held their hand over it. The pupillometer has excellent resolution (mean error=0.052 mm; 99% CI=0.048–0.056; NeurOptics data, N=655).
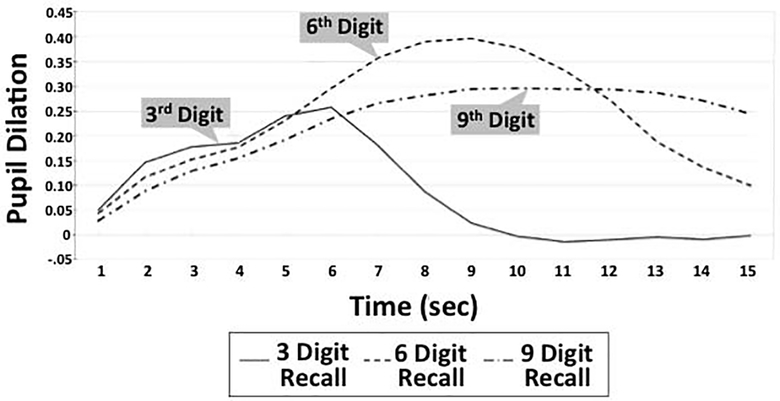
Pupillary responses were recorded during blocks of trials of 3 (low load), 6 (moderate/near capacity load), and 9 (high/overload) digits presented aurally at the rate of 1 per second. Stimuli were presented on a laptop computer at ~85 decibels. Participants heard “Ready” 1 second before the first digit and “Repeat” 1 second after the last digit. Experimenters initiated pupillary response recording when the word “Ready” was presented. We recorded from the right eye (72.4% of the time) unless the participant had had an injury or medical problem in that eye. Each trial was inspected for artifacts in a graphic display on the device. Trials were administered until 2 clean trials were recorded or 4 trials were attempted per digit span condition. We averaged trials within each condition and averaged pupil diameter samples for each second of recording (30 per second), corresponding to the presentation of digits at 1-second intervals. The primary dependent variable was the pupillary response score: average pupil size during the 1-second window immediately following presentation of the last digit minus average pupil size at baseline (i.e., the first 167ms of each trial, which is prior to stimulus presentation). These difference scores remove individual differences in tonic pupil size. Figure 2 shows a sample pupil response waveform.
Fig. 2.
Sample pupillary response waveform during digit span at 3 cognitive loads. Pupil dilation is calculated as mm change from baseline.
2.4. Genotyping methods
These methods are described in detail elsewhere (Logue et al., 2018). Genome-wide genotyping was conducted on individual twins, with one randomly selected twin from each MZ pair at deCODE (Reykjavik, Iceland) with Illumina HumanOmniExpress-24 v1.0A beadchips. GenomeStudio software indicated that the average call rate was 0.996. We performed cleaning and quality control with PLINK v1.9 (Chang et al., 2015). Single nucleotide polymorphisms (SNPs) with >5% missing data or Hardy-Weinberg equilibrium P-values<10−6 were excluded. Relationships and zygosity were confirmed by PLINK’s-genome procedure.
Ancestry was confirmed by SNPweights (Chen et al., 2013) and a principal components (PCs) analysis performed in PLINK v1.9 in conjunction with 1000 Genomes Phase 3 reference data (1000 Genomes Project Consortium et al., 2015). Weights for PCs were computed from 100,000 randomly chosen common (minor allele frequency [MAF]>5%) markers based on 1000 Genomes data and then applied to the VETSA sample. Outliers from the EUR population (1000 Genomes European-ancestry super population) cluster were excluded from the genetically-identified VETSA white non-Hispanic cohort. The remaining white non-Hispanic participants had >89% European ancestry as estimated by SNPweights. PCs for use as covariates to control for potential population substructure within white non-Hispanic participants were recomputed based on 100,000 randomly chosen common markers.
Imputation was performed using MiniMac (Fuchsberger et al., 2015; Howie et al., 2012) at the Michigan Imputation Server (https://imputationserver.sph.umich.edu). The 1,000 genomes phase 3 EUR data were used as a haplotype reference panel. Only one randomly chosen individual in each genotyped MZ pair was submitted for imputation. The resulting imputed genotypes were then applied to the co-twin. The final sample with available imputation data included 1,329 individuals.
2.4.1. AD-PRS calculation and APOE genotyping
The AD-PRS was computed from summary data of an AD GWAS meta-analysis (Lambert et al., 2013). It is a weighted average of VETSA sample additive imputed SNP dosages with log-odds ratios for each SNP estimated in the GWAS used as the weights. We excluded rare SNPs (MAF<1%) and SNPs with poor imputation quality (R2<0.5) from the calculation. We trimmed the remaining SNPs for linkage disequilibrium (LD) using PLINK’s clumping procedure (r2 threshold of 0.2 in a 500 kb window) based on LD patterns in the 1000 Genomes EUR cohort. AD-PRSs were computed by PLINK v1.9 using 6 P-value thresholds: P<0.05, 0.10, 0.20, 0.30, 0.40, 0.50. In addition, we directly genotyped APOE as described previously (Schultz et al., 2008). The number of SNPs included at different thresholds has been documented in a prior publication (Logue et al., 2018). In our study of MCI and in studies of AD, the P<0.50 threshold provided the best case-control discrimination (Escott-Price et al., 2017a; Escott-Price et al., 2015; Logue et al., 2018). We, therefore, used the P<0.50 threshold in the present study.
2.5. Statistical analysis
2.5.1. Heritability and estimation of genetic and environmental influences
In the classical twin design, variance of a phenotype is separated into proportions attributed to additive genetic (A), common environmental (C), and unique environmental (E) influences. C influences are environmental factors that make twins in a pair similar to one another; E influences are environmental factors that make twins in a pair different from one another, including measurement error (Eaves et al., 1978; Neale and Cardon, 1992). Additive genetic influences are assumed to correlate 1.0 between MZ twins, and 0.50 between DZ twins who on average share 50% of their segregating genes. C influences are assumed to correlate 1.0 between members of a pair regardless of zygosity. E influences are, by definition, uncorrelated between members of a pair. Heritability is the proportion of total variance attributed to additive genetic influences.
Extending to the multivariate case, we examined the relative contribution of the genetic and environmental influences on pupil dilation responses at the 3 cognitive loads and the covariance between these measures by fitting a Cholesky decomposition to the data. The purpose was to determine the degree to which covariance between individual differences at the 3 cognitive loads can be explained by common versus distinct continua of liability. We began by fitting a Cholesky that included the A, C, and E effects, then tested if the A or C components could be removed without any change in model fit. We tested model fit using the likelihood-ratio chi-square test (LRT), which is the difference in the −2 log likelihood (−2LL) of the model in question relative to the full saturated model. Nonsignificant LRT values (P>.05) indicate that a reduced model does not have a significantly worse fit relative to the comparison. Additionally, we used the Akaike Information Criterion (AIC) as an indicator of goodness-of-fit; smaller values represent a better balance between goodness-of-fit and parsimony (Akaike, 1987). Analyses were conducted using the raw data option of the maximum-likelihood based structural equation modeling software OpenMx (Boker et al., 2011; Neale et al., 2015).
Residual pupillary response scores were used in the biometrical models, after adjustment for age, pupillometry device (4 of the same devices were used), and medications with anticholinergic properties. Relevant medications and their rankings for degree of anticholinergic properties have been documented previously (Granholm et al., 2017).
2.5.2. AD-PRS
These analyses were conducted using linear mixed effects models (SAS Proc Mixed, version 9.4; SAS Institute Inc., 2013) accounting for the correlated nature of the twin data by including family (i.e., twin pair) as a random effect. The AD-PRS was standardized prior to analysis. We included the first 3 PCs, age, pupillometry device, and medications with anticholinergic properties as covariates. We also compared the upper versus lower quartile of the AD-PRS distribution. To determine effects of the AD-PRS after accounting for APOE, we performed additional analyses including directly genotyped APOE-ε2 and APOE-ε4. Each was coded for presence/absence of at least one ε2 or ε4 allele, respectively. Results were based on type III tests of fixed effects.
3. Results
The full Cholesky provided a good fit to the data (−2LL=4800.15, df=1570, AIC=1660.14). Two C estimates accounted for ≤1% of variance. A reduced Cholesky with those parameters set to zero resulted in minimal change in fit (−2LL=4800.43, df=1575, AIC=1650.43, LRT=.29, df=5, P>.999). All 3 pupillary response measures were significantly heritable (h2=0.30–0.36); the remaining variance was primarily accounted for by unique environmental influences (Table 2). The unstandardized variance components for the reduced Cholesky also show that the genetic and the total variance in pupillary responses increased as cognitive load increased (Table 2). However, heritabilities changed little with increasing cognitive load because genetic and unique environmental variances were both increasing.
Table 2.
Variance components of pupillary dilation response measures
| Standardized Variance Components | |||
| Measure | A (95% CI) | C (95% CI) | E (95% CI) |
| Full Cholesky | |||
| Dilation at 3 Digits | .36 (.10;.52) | .00 (.00;.20) | .64 (.48;.83) |
| Dilation at 6 Digits | .33 (.05;.59) | .14 (.00;.38) | .53 (.38;.74) |
| Dilation at 9 Digits | .36 (.06;.54) | .01 (.00;.23) | .63 (.46;.84) |
| Reduced Cholesky | |||
| Dilation at 3 Digits | .36 (.17;.52) | ---- | .64 (.48;.83) |
| Dilation at 6 Digits | .30 (.10;.60) | .17 (.00;.32) | .53 (.38;.73) |
| Dilation at 9 Digits | .37 (.17;.54) | ---- | .63 (.46;.83) |
| Unstandardized Variance Components | |||
| Measure | A | C | E |
| Reduced Cholesky | |||
| Dilation at 3 Digits | .36 | ---- | .65 |
| Dilation at 6 Digits | .57 | .32 | 1.01 |
| Dilation at 9 Digits | .76 | ---- | 1.26 |
Key: A, additive genetic influences; C, common/shared environmental influences; E, unique environmental influences; CI, confidence interval.
Table 3 shows the correlations among pupillary response measures derived from the reduced Cholesky. Phenotypic correlations, which represent the total shared variance between measures, were moderate (rP=0.42–0.60). Genetic correlations, which represent only the shared genetic variance between measures (Neale and Cardon, 1992), were substantially higher (rG=0.73–0.99). The high genetic correlations suggest that genetic influences affecting dilation at varying digit lengths are driven primarily by a single common factor. However, 2 genetic correlations were significantly different from 1.0, indicating that they are not entirely influenced by the same genes. Because unselected samples are thought to provide more unbiased heritability estimates, we also provide the full sample (n=1119) Cholesky and correlation results, which were very similar (Supplementary Tables 1 and 2). However, as already noted, we focus primarily on the subset of individuals with span capacities of 5–7 because of the very different meaning of the task for people at the extremes of span capacity.
Table 3.
Phenotypic, genetic, and unique environmental correlations among pupillary dilation response measures
| Measures | 3 digits | 6 digits | 9 digits |
|---|---|---|---|
| Phenotypic correlations | |||
| Dilation at 3 digits | 1.00 | ||
| Dilation at 6 digits | .42 (.35 ; .49) | 1.00 | |
| Dilation at 9 digits | .42 (.35 ; .49) | .60 (.54 ; .65) | 1.00 |
| Genetic correlations | |||
| Dilation at 3 digits | 1.00 | ||
| Dilation at 6 digits | .99 (.58 ; 1.0) | 1.00 | |
| Dilation at 9 digits | .73 (.42 ; .96) | .63 (.18 ; .93) | 1.00 |
| Unique environmental correlations | |||
| Dilation at 3 digits | 1.00 | ||
| Dilation at 6 digits | .17 (−.26 ; .36) | 1.00 | |
| Dilation at 9 digits | .24 (.06 ; .42) | .67 (.53 ; .77) | 1.00 |
Numbers in parentheses are the 95% confidence intervals. All estimates were derived from the reduced trivariate Cholesky decomposition.
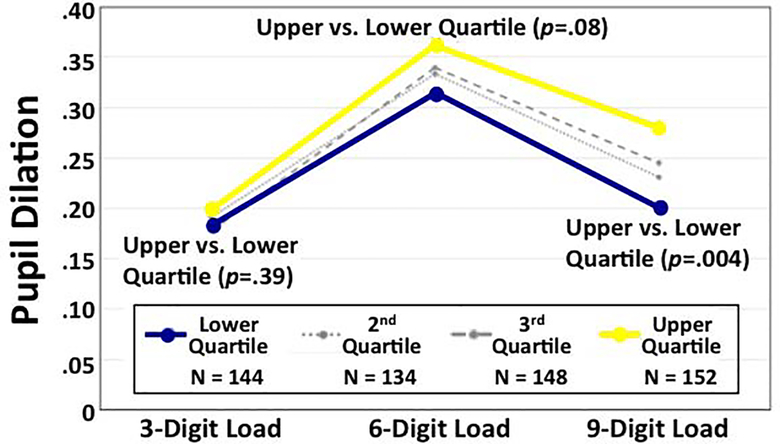
The AD-PRS was significantly correlated with pupil dilation response during the 9-digit recall condition (r=0.10, P<0.03; Table 4). As expected, results for the cognitively normal sample including participants with the full range of span capacities were weaker, although in the same direction (Supplementary Table 3). The difference between the upper and lower quartiles quartile of the AD-PRS distribution increased as the cognitive load increased (Figure 3). The upper quartile had significantly larger pupil responses during 9-digit recall (Cohen’s d=0.36, P<0.005; Table 5), and this comparison was at a trend level for the 6-digit recall (d=0.22, P<0.08). These sets of results held up after including maximum span capacity as a covariate (r=0.09, p<0.03; Supplementary Table 4) and after controlling for depression and history of head injury (r=0.09, p<0.04; Supplementary Table 5). After controlling for presence/absence of directly genotyped APOE-ε2 and APOE-ε4, the AD-PRS was still significantly correlated with pupil dilation responses during the high cognitive load condition (r=0.11, p<0.02; Supplementary Table 6). Neither APOE variant was associated with pupil dilation responses.
Table 4.
Association of Alzheimer’s disease polygenic risk score with pupillary dilation response
| Digit Span Load | Estimate | SE | DF | t | p | r |
|---|---|---|---|---|---|---|
| 3 Digits (n=537) | 0.003 | 0.007 | 139 | 0.42 | .67 | .02 |
| 6 Digits (n=530) | 0.014 | 0.010 | 135 | 1.42 | .16 | .06 |
| 9 Digits (n=521) | 0.023 | 0.010 | 130 | 2.18 | .03 | .10 |
Covariates include age, the first 3 principal components from the genome-wide genotyping data, pupillometry device, and total number of medications with anticholinergic properties. Data were restricted to cognitively normal individuals with a maximum digit span of 5–7 digits. Ns vary due to missing data for particular variables.
Fig. 3.
Pupillary dilation response during digit span tasks: Upper vs. lower quartiles of the AD-PRS distribution. AD-PRS, Alzheimer’s disease polygenic risk score. Pupil dilation is calculated as mm change from baseline.
Table 5.
Association of Alzheimer’s disease polygenic risk score (upper vs. lower quartile) with pupillary dilation response
| Digit Span Load | Estimate | SE | DF | t | p | d |
|---|---|---|---|---|---|---|
| 3 Digits (n=272) | −0.017 | 0.020 | 137 | −0.86 | .39 | .10 |
| 6 Digits (n=267) | −0.048 | 0.027 | 133 | −1.79 | .08 | .22 |
| 9 Digits (n=264) | −0.080 | 0.028 | 128 | −2.88 | .005 | .36 |
Covariates include age, the first 3 principal components from the genome-wide genotyping data; pupillometry device, and total number of medications with anticholinergic properties. Data were restricted to cognitively normal individuals with a maximum digit span of 5–7 digits. Ns vary due to missing data for particular variables. Results presented represent the difference between the upper and lower quartiles of the AD-PRS distribution.
We compared pupillary dilation responses that were recorded from the left versus right eye. There was no difference at the 6-digit load (p=0.12) or the 9-digit load (p=0.46), but there was significantly greater dilation in the right eye at the 3-digit load (F(1,365)=7.06, p=.008). As a check, we re-ran all of the analyses with the eye from which pupillary responses were recorded as a covariate. These results are not reported here as there were no meaningful changes in the findings, and all significant results remained significant.
4. Discussion
To our knowledge, this is the first evidence of the heritability of task-relevant pupillary dilation responses. High genetic correlations suggest that individual differences in dilation during different cognitive loads are driven primarily by a single common factor or underlying continuum of liability. We then showed that CN individuals at greater genetic risk for AD—based on the AD-PRS—had significantly greater pupil dilation when cognitive demand was high. The effect size comparing the upper and lower quartiles of the AD-PRS distribution was d=.36. Consistent with an underlying continuum of liability, there was an increasing effect size with increasing cognitive load.
We previously observed a wide distribution of pupillary responses in CN individuals, and hypothesized that those with the highest pupil dilation would be at highest risk for progressing to MCI and AD (Granholm et al., 2017). Although we do not yet know who will develop these disorders, our results support this hypothesis because those who required the greatest effort as cognitive load increased also tend to be those at highest genetic risk based on the AD-PRS. The minimal variation in actual performance in this sample and additional analyses controlling for maximum span show that risk was associated with effort needed rather than task performance. Thus, these results provide proof of concept that pupillary dilation responses during a cognitive task—a brief, low-cost, low-invasive assessment—might be a useful additional risk indicator for identifying participants for clinical trials or other research on determinants of onset and progression of AD. The present results and our previous finding that pupillary responses during digit span differentiated CN individuals from those with amnestic MCI suggest that this measure has potential as an adjunctive screening tool, probably in combination with other biomarkers. However, more work needs to be done before its utility in improving screening for AD risk can be determined.
To ensure relatively comparable difficulty level and performance across participants, we only included participants with max spans of 5–7 digits. For individuals with max spans >7, 9 digits is not as much of an overload, and for individuals with a max span of <5 digits, 6 digits is closer to overload. These distinctions are important because, relative to individuals with lower ability, individuals with greater ability dilate less at low loads but more in higher load conditions (Ahern and Beatty, 1979; Granholm et al., 2017; van der Meer et al., 2010). It is, therefore, important to examine dilation relative to individual ability level. Put another way, at a given cognitively load, pupil responses for people with very high or very low span capacities probably reflect different processes. Thus, it was not unexpected that we observed weaker results for the full sample of cognitively normal individuals.
Here we used pre-set cognitive loads because it was important in our initial work (Granholm et al., 2017) to show that pupil responses differed in a systematic way as a function of capacity and processing load. Having demonstrated proof of concept, it will be necessary to implement idiographic approaches for meaningful future comparison of individuals across the full range of span capacities in which cognitive loads are tailored to each individual’s capacity (e.g., defining high load as 2 digits above each individual’s maximum span). Finally, we chose digit span, in part, due to practical constraints of the pupillometry device. However, we have successfully piloted pupil response on a new device with which we can assess episodic memory. Thus, proof of concept demonstrated here will be fully applicable to future studies using idiographic approaches with more AD-relevant episodic memory tests.
Here we acknowledge some limitations. Although this was a community-based sample, it was all male and largely white, non-Hispanic. All had past military service, but the large majority was non-combat-related. Generalization to women or racial/ethnic minorities remains to be determined. We would expect pupillary dilation responses to be heritable at younger ages, given that studies of younger individuals have shown substantial variability of pupillary responses and inverse associations between pupillary dilation and cognitive capacity (Ahern and Beatty, 1979; Beatty, 1982; Granholm et al., 1996; Kahneman and Beatty, 1966; van der Meer et al., 2010). However, we do not know the extent to which pupillary responses at younger ages may be influenced by the same or different genes. It is possible, for example, that more AD risk genes are associated with pupillary responses during mid- and later life compared with earlier life. We also do not know if the highest cognitive load would best predict risk in other age groups. However, if one’s interest is in biomarkers of early risk for cognitive decline or AD, it is middle-aged adults that may be most appropriate. It will be of interest to determine how AD biomarkers (currently being assessed in this sample) are related to pupillary responses, and if pupillary responses might in some cases detect risk before currently defined Aß and tau thresholds are reached.
5. Conclusion
Pupillary dilation responses are largely driven by the LC-NE system (Samuels and Szabadi, 2008; Wilhelm et al., 1999), an important modulator of cognitive function (Aston-Jones and Cohen, 2005; Sara, 2009). The LC is also an early site of tau deposition. This led to our previous work comparing CN and MCI groups, which supports pupillary response as a potential psychophysiological biomarker of risk for MCI and AD (Granholm et al., 2017). Here we showed that pupillary dilation responses are associated with AD risk genes. Given evidence linking pupillary responses, LC, and tau, the association between the AD-PRS and pupillary response provides additional evidence that is consistent with pupillary responses as a genetically-mediated MCI/AD biomarker. Although the utility of pupillary responses recorded during cognitive tasks remains to be determined, the results provide proof of concept that this brief, low-cost, low-invasive test may, in combination with other measures, aid in first-line screening to identify adults at increased genetic risk for AD while they are still cognitively normal. Identifying the specific genes associated with the pupillary response factors may improve understanding of the functioning of the LC-NE system and of genetically-mediated factors affecting risk for MCI and AD.
Supplementary Material
Highlights.
Locus coeruleus (LC) function may point to early Alzheimer’s disease (AD) risk
Pupil dilation response during cognitive tasks is thought to reflect LC function.
Pupil response in cognitively normal adults is associated with AD polygenic risk
Pupil response may improve early screening before cognitive performance declines
Acknowledgments
This work was supported by the National Institute on Aging grants R01AG050595 (W.S.K., M.J.L., C.E.F.), R01AG022381 (W.S.K.), P01AG055367 and R01AG059329 (C.E.F. [sub-PI]), K08 AG047903 (M.S.P.), AG054509 (E.L.G.), Research Council of Norway 223273 (OA.A.), and Stiftelsen KG Jebsen (O.A.A.), KL2TR00144 and P50AG005131 (J.A.E [sub-award]). This material was, in part, the result of work supported with resources of the VA San Diego Center of Excellence for Stress and Mental Health. The Cooperative Studies Program of the U.S. Department of Veterans Affairs also provided financial support for development and maintenance of the Vietnam Era Twin Registry. The content is solely the responsibility of the authors and does not necessarily represent official views of the National Institutes of Health or the Department of Veterans Affairs. We acknowledge the continued cooperation and participation of the members of the VET Registry and their families.
Declaration of Interest
Dr. Dale is a Founder of and holds equity in CorTechs Labs, Inc, and serves on its Scientific Advisory Board. He is a member of the Scientific Advisory Board of Human Longevity, Inc. and receives funding through research agreements with General Electric Healthcare and Medtronic, Inc. The terms of these arrangements have been reviewed and approved by UCSD in accordance with its conflict of interest policies. The remaining authors have no declarations of interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1000 Genomes Project Consortium, Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, Marchini JL, McCarthy S, McVean GA, Abecasis GR, 2015. A global reference for human genetic variation. Nature 526(7571), 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahern S, Beatty J, 1979. Pupillary responses during information processing vary with Scholastic Aptitude Test scores. Science 205(4412), 1289–1292. [DOI] [PubMed] [Google Scholar]
- Akaike H, 1987. Factor analysis and AIC. Psychometrika 52, 317–332. [Google Scholar]
- Alnaes D, Sneve MH, Espeseth T, Endestad T, van de Pavert SH, Laeng B, 2014. Pupil size signals mental effort deployed during multiple object tracking and predicts brain activity in the dorsal attention network and the locus coeruleus. J Vis 14(4). [DOI] [PubMed] [Google Scholar]
- Alzheimer’s Association, 2018. 2018 Alzheimer’s disease facts and figures. Alzheimers Dement. 14(3), 367–429. [Google Scholar]
- Aston-Jones G, Cohen JD, 2005. An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Annu. Rev. Neurosci 28, 403–450. [DOI] [PubMed] [Google Scholar]
- Beatty J, 1982. Task-evoked pupillary responses, processing load, and the structure of processing resources. Psychol. Bull 91, 276–292. [PubMed] [Google Scholar]
- Boker S, Neale MC, Maes H, Wilde M, Spiegel M, Brick T, Spies J, Estabrook R, Kenny S, Bates T, Mehta P, J., F., 2011. OpenMx: An open source extended structural equation modeling framework. Psychometrika 76, 306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, 2011. The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol 121(2), 171–181. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, 2012. Where, when, and in what form does sporadic Alzheimer’s disease begin? Curr. Opin. Neurol 25(6), 708–714. [DOI] [PubMed] [Google Scholar]
- Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ, 2015. Second-generation PLINK: Rising to the challenge of larger and richer datasets. Gigascience 4, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Pollack S, Hunter DJ, Hirschhorn JN, Kraft P, Price AL, 2013. Improved ancestry inference using weights from external reference panels. Bioinformatics 29(11), 1399–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewett DV, Lee T-H, Greening S, Ponzio A, Margalit E, Mather M, 2016. Neuromelanin marks the spot: Identifying a locus coeruleus biomarker of cognitive reserve in healthy aging. Neurobiol. Aging 37, 117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder E, Saunders A, Strittmatter W, Schmechel D, Gaskell P, Small G, Roses A, Haines J, Pericak-Vance M, 1993. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261(5123), 921–923. [DOI] [PubMed] [Google Scholar]
- Coull JT, Buchel C, Friston KJ, Frith CD, 1999. Noradrenergically mediated plasticity in a human attentional neuronal network. Neuroimage 10(6), 705–715. [DOI] [PubMed] [Google Scholar]
- de Leon MJ, Pirraglia E, Osorio RS, Glodzik L, Saint-Louis L, Kim HJ, Fortea J, Fossati S, Laska E, Siegel C, Butler T, Li Y, Rusinek H, Zetterberg H, Blennow K, Alzheimer’s Disease Neuroimaging I, National Alzheimer’s Coordinating C, 2018. The nonlinear relationship between cerebrospinal fluid Aß42 and tau in preclinical Alzheimer’s disease. PLoS One 13(2), e0191240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duyckaerts C, Braak H, Brion J-P, Buée L, Del Tredici K, Goedert M, Halliday G, Neumann M, Spillantini MG, Tolnay M, Uchihara T, 2015. PART is part of Alzheimer disease. Acta Neuropathol. (Berl) 129(5), 749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaves LJ, Last KA, Young PA, Martin NG, 1978. Model-fitting approaches to the analysis of human behavior. Heredity 41, 249–320. [DOI] [PubMed] [Google Scholar]
- Ehrenberg AJ, Nguy AK, Theofilas P, Dunlop S, Suemoto CK, Di Lorenzo Alho AT, Leite RP, Diehl Rodriguez R, Mejia MB, Rub U, Farfel JM, de Lucena Ferretti-Rebustini RE, Nascimento CF, Nitrini R, Pasquallucci CA, Jacob-Filho W, Miller B, Seeley WW, Heinsen H, Grinberg LT, 2017. Quantifying the accretion of hyperphosphorylated tau in the locus coeruleus and dorsal raphe nucleus: The pathological building blocks of early Alzheimer’s disease. Neuropathol. Appl. Neurobiol 43(5), 393–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman JA, Panizzon MS, Hagler DJ Jr., Eyler LT, Granholm EL, Fennema-Notestine C, Lyons MJ, McEvoy LK, Franz CE, Dale AM, Kremen WS, 2017. Task-evoked pupil dilation and BOLD variance as indicators of locus coeruleus dysfunction. Cortex 97, 60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escott-Price V, Myers AJ, Huentelman M, Hardy J, 2017a. Polygenic risk score analysis of pathologically confirmed Alzheimer disease. Ann. Neurol 82(2), 311–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escott-Price V, Shoai M, Pither R, Williams J, Hardy J, 2017b. Polygenic score prediction captures nearly all common genetic risk for Alzheimer’s disease. Neurobiol. Aging 49, 214.e217–214.e211. [DOI] [PubMed] [Google Scholar]
- Escott-Price V, Sims R, Bannister C, Harold D, Vronskaya M, Majounie E, Badarinarayan N, Gerad/Perades, consortia, I., Morgan K, Passmore P, Holmes C, Powell J, Brayne C, Gill M, Mead S, Goate A, Cruchaga C, Lambert JC, van Duijn C, Maier W, Ramirez A, Holmans P, Jones L, Hardy J, Seshadri S, Schellenberg GD, Amouyel P, Williams J, 2015. Common polygenic variation enhances risk prediction for Alzheimer’s disease. Brain 138(Pt 12), 3673–3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotiou DF, Stergiou V, Tsiptsios D, Lithari C, Nakou M, Karlovasitou A, 2009. Cholinergic deficiency in Alzheimer’s and Parkinson’s disease: Evaluation with pupillometry. Int. J. Psychophysiol 73(2), 143–149. [DOI] [PubMed] [Google Scholar]
- Fotiou F, Fountoulakis KN, Tsolaki M, Goulas A, Palikaras A, 2000. Changes in pupil reaction to light in Alzheimer’s disease patients: A preliminary report. Int. J. Psychophysiol 37(1), 111–120. [DOI] [PubMed] [Google Scholar]
- Fuchsberger C, Abecasis GR, Hinds DA, 2015. minimac2: faster genotype imputation. Bioinformatics 31(5), 782–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Torraville SE, Mukherjee B, Walling SG, Martin GM, Harley CW, Yuan Q, 2019. An experimental model of Braak’s pretangle proposal for the origin of Alzheimer’s disease: the role of locus coeruleus in early symptom development. Alzheimers Res. Ther 11(1), Article number 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilzenrat MS, Nieuwenhuis S, Jepma M, Cohen JD, 2010. Pupil diameter tracks changes in control state predicted by the adaptive gain theory of locus coeruleus function. Cogn Affect Behav Neurosci 10(2), 252–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golde TE, Schneider LS, Koo EH, 2011. Anti-abeta therapeutics in Alzheimer’s disease: The need for a paradigm shift. Neuron 69, 203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granholm E, Asarnow RF, Sarkin AJ, Dykes KL, 1996. Pupillary responses index cognitive resource limitations. Psychophysiology 33(4), 457–461. [DOI] [PubMed] [Google Scholar]
- Granholm EL, Panizzon MS, Elman JA, Jak AJ, Hauger RL, Bondi MW, Lyons MJ, Franz CE, Kremen WS, 2017. Pupillary responses as a biomarker of early risk for Alzheimer’s disease. J Alzheimers Dis 56(4), 1419–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämmerer D, Callaghan MF, Hopkins A, Kosciessa J, Betts M, Cardenas-Blanco A, Kanowski M, Weiskopf N, Dayan P, Dolan RJ, Düzel E, 2018. Locus coeruleus integrity in old age is selectively related to memories linked with salient negative events. Proceedings of the National Academy of Sciences 115(9), 2228–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howie B, Fuchsberger C, Stephens M, Marchini J, Abecasis GR, 2012. Fast and accurate genotype imputation in genome-wide association studies through pre-phasing. Nat. Genet. 44(8), 955–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jak AJ, Bondi MW, Delano-Wood L, Wierenga C, Corey-Bloom J, Salmon DP, Delis DC, 2009. Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am. J. Geriatr. Psychiatry 17(5), 368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, Li Y, Kalwani Rishi M., Gold Joshua I., 2016. Relationships between pupil diameter and neuronal activity in the locus coeruleus, colliculi, and cingulate cortex. Neuron 89(1), 221–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahneman D, Beatty J, 1966. Pupil diameter and load on memory. Science 154(3756), 1583–1585. [DOI] [PubMed] [Google Scholar]
- Kaup AR, Toomey R, Bangen KJ, Delano-Wood L, Yaffe K, Panizzon MS, Lyons MJ, Franz CE, Kremen WS, 2019. Interactive effect of traumatic brain injury and psychiatric symptoms on cognition among late middle-aged men: Findings from the Vietnam Era Twin Study of Aging . J. Neurotrauma 36(2), 338–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss MC, 1986. Pupillary dilation as an index of central nervous system alpha 2-adrenoceptor activation. J. Pharmacol. Methods 15(1), 1–19. [DOI] [PubMed] [Google Scholar]
- Kremen WS, Franz CE, Lyons MJ, 2013. VETSA: The Vietnam Era Twin Study of Aging. Twin Res. Hum. Genet 16, 399–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen WS, Jak AJ, Panizzon MS, Spoon KM, Franz CE, Thompson WK, Jacobson KC, Vasilopoulos T, Vuoksimaa E, Xian H, Toomey R, Lyons MJ, 2014. Early identification and heritability of mild cognitive impairment. Int. J. Epidemiol 43(2), 600–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen WS, Thompson-Brenner H, Leung YM, Grant MD, Franz CE, Eisen SA, Jacobson KC, Boake C, Lyons MJ, 2006. Genes, environment, and time: The Vietnam Era Twin Study of Aging (VETSA). Twin Res. Hum. Genet 9(6), 1009–1022. [DOI] [PubMed] [Google Scholar]
- Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, DeStafano AL, Bis JC, Beecham GW, Grenier-Boley B, Russo G, Thorton-Wells TA, Jones N, Smith AV, Chouraki V, Thomas C, Ikram MA, Zelenika D, Vardarajan BN, Kamatani Y, Lin CF, Gerrish A, Schmidt H, Kunkle B, Dunstan ML, Ruiz A, Bihoreau MT, Choi SH, Reitz C, Pasquier F, Cruchaga C, Craig D, Amin N, Berr C, Lopez OL, De Jager PL, Deramecourt V, Johnston JA, Evans D, Lovestone S, Letenneur L, Moron FJ, Rubinsztein DC, Eiriksdottir G, Sleegers K, Goate AM, Fievet N, Huentelman MW, Gill M, Brown K, Kamboh MI, Keller L, Barberger-Gateau P, McGuiness B, Larson EB, Green R, Myers AJ, Dufouil C, Todd S, Wallon D, Love S, Rogaeva E, Gallacher J, St George-Hyslop P, Clarimon J, Lleo A, Bayer A, Tsuang DW, Yu L, Tsolaki M, Bossu P, Spalletta G, Proitsi P, Collinge J, Sorbi S, Sanchez-Garcia F, Fox NC, Hardy J, Deniz Naranjo MC, Bosco P, Clarke R, Brayne C, Galimberti D, Mancuso M, Matthews F, European Alzheimer’s Disease I, Genetic, Environmental Risk in Alzheimer’s, D., Alzheimer’s Disease Genetic, C., Cohorts for, H., Aging Research in Genomic, E., Moebus S, Mecocci P, Del Zompo M, Maier W, Hampel H, Pilotto A, Bullido M, Panza F, Caffarra P, Nacmias B, Gilbert JR, Mayhaus M, Lannefelt L, Hakonarson H, Pichler S, Carrasquillo MM, Ingelsson M, Beekly D, Alvarez V, Zou F, Valladares O, Younkin SG, Coto E, Hamilton-Nelson KL, Gu W, Razquin C, Pastor P, Mateo I, Owen MJ, Faber KM, Jonsson PV, Combarros O, O’Donovan MC, Cantwell LB, Soininen H, Blacker D, Mead S, Mosley TH Jr., Bennett DA, Harris TB, Fratiglioni L, Holmes C, de Bruijn RF, Passmore P, Montine TJ, Bettens K, Rotter JI, Brice A, Morgan K, Foroud TM, Kukull WA, Hannequin D, Powell JF, Nalls MA, Ritchie K, Lunetta KL, Kauwe JS, Boerwinkle E, Riemenschneider M, Boada M, Hiltuenen M, Martin ER, Schmidt R, Rujescu D, Wang LS, Dartigues JF, Mayeux R, Tzourio C, Hofman A, Nothen MM, Graff C, Psaty BM, Jones L, Haines JL, Holmans PA, Lathrop M, Pericak-Vance MA, Launer LJ, Farrer LA, van Duijn CM, Van Broeckhoven C, Moskvina V, Seshadri S, Williams J, Schellenberg GD, Amouyel P, 2013. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat. Genet 45(12), 1452–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T-H, Greening SG, Ueno T, Clewett D, Ponzio A, Sakaki M, Mather M, 2018. Arousal increases neural gain via the locus coeruleus–noradrenaline system in younger adults but not in older adults. Nature Human Behaviour 2(5), 356–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logue MW, Panizzon MS, Elman JA, Gillespie NA, Hatton SN, Gustavson DE, Andreassen OA, Dale AM, Franz CE, Lyons MJ, Neale MC, Reynolds CA, Tu X, Kremen WS, 2018. Use of an Alzheimer’s disease polygenic risk score to identify mild cognitive impairment in adults in their 50s. Mol. Psychiatry 24, 421–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons MJ, Panizzon MS, Liu W, McKenzie R, Bluestone NJ, Grant MD, Franz CE, Vuoksimaa EP, Toomey R, Jacobson KC, Reynolds CA, Kremen WS, Xian H, 2017. A longitudinal twin study of general cognitive ability over four decades. Dev. Psychol 53(6), 1170–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons MJ, York TP, Franz CE, Grant MD, Eaves LJ, Jacobson KC, Schaie KW, Panizzon MS, Boake C, Xian H, Toomey R, Eisen SA, Kremen WS, 2009. Genes determine stability and the environment determines change in cognitive ability during 35 years of adulthood. Psychol. Sci 20, 1146–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maass A, Lockhart SN, Harrison TM, Bell RK, Mellinger T, Swinnerton K, Baker SL, Rabinovici GD, Jagust WJ, 2018. Entorhinal tau pathology, episodic memory decline, and neurodegeneration in aging. The Journal of Neuroscience 38(3), 530–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Harley CW, 2016. The locus coeruleus: Essential for maintaining cognitive function and the aging brain. Trends Cogn Sci 20(3), 214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy PR, O’Connell RG, O’Sullivan M, Robertson IH, Balsters JH, 2014. Pupil diameter covaries with BOLD activity in human locus coeruleus. Hum. Brain Mapp. 35(8), 4140–4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale MC, Cardon LR, 1992. Methodology for genetic studies of twins and families. Kluwer, Dordrecht, The Netherlands. [Google Scholar]
- Neale MC, Hunter MD, Pritikin JN, Zahery M, Brick TR, Kirkpatrick RM, Estabrook R, Bates TC, Maes HH, Boker SM, 2015. OpenMx 2.0: Extended structural equation and statistical modeling. Psychometrika 81, 535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips MA, Szabadi E, Bradshaw CM, 2000. Comparison of the effects of clonidine and yohimbine on spontaneous pupillary fluctuations in healthy human volunteers. Psychopharmacology (Berl). 150(1), 85–89. [DOI] [PubMed] [Google Scholar]
- Prettyman R, Bitsios P, Szabadi E, 1997. Altered pupillary size and darkness and light reflexes in Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 62(6), 665–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS, 1977. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement 1, 385–401. [Google Scholar]
- Raizada RD, Poldrack RA, 2007. Challenge-driven attention: interacting frontal and brainstem systems. Front. Hum. Neurosci 1, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riediger M, Li S-C, Lindenberger U, 2006. Selection, optimization, and compensation as developmental mechanisms of adaptive resource allocation: Review and preview, in: Birren JE, Schaie KW (Eds.), Handbook of the Psychology of Aging. Burlington, MA, Amsterdam, pp. 289–313. [Google Scholar]
- Samuels ER, Szabadi E, 2008. Functional neuroanatomy of the noradrenergic locus coeruleus: its roles in the regulation of arousal and autonomic function part II: Physiological and pharmacological manipulations and pathological alterations of locus coeruleus activity in humans. Current Neuropharmacology 6(3), 254–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara SJ, 2009. The locus coeruleus and noradrenergic modulation of cognition. Nature Reviews. Neuroscience 10(3), 211–223. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc., 2013. SAS OnlineDoc 9.4 SAS Institute, Carey, NC. [Google Scholar]
- Schoeneborn CA, Heyman KM, 2009. Health characteristics of adults aged 55 years and over: United States, 2004–2007, National Health Statistics Reports. National Center for Health Statistics, Hyattsville, MD, pp. 1–31. [PubMed] [Google Scholar]
- Schultz MR, Lyons MJ, Franz CE, Grant MD, Boake C, Jacobson KC, Xian H, Schellenberg GD, Eisen SA, Kremen WS, 2008. Apolipoprotein E genotype and memory in the sixth decade of life. Neurology 70, 1771–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR Jr., Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH, 2011. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Arenaza-Urquijo EM, Bartres-Faz D, Belleville S, Cantilon M, Chetelat G, Ewers M, Franzmeier N, Kempermann G, Kremen WS, Okonkwo O, Scarmeas N, Soldan A, Udeh-Momoh C, Valenzuela M, Vemuri P, Vuoksimaa E, Reserve R, Protective Factors PIAED, Conceptual Frameworks W, 2018. Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer E, Beyer R, Horn J, Foth M, Bornemann B, Ries J, Kramer J, Warmuth E, Heekeren HR, Wartenburger I, 2010. Resource allocation and fluid intelligence: Insights from pupillometry. Psychophysiology 47(1), 158–169. [DOI] [PubMed] [Google Scholar]
- Walle KM, Nordvik JE, Espeseth T, Becker F, Laeng B, 2019. Multiple object tracking and pupillometry reveal deficits in both selective and intensive attention in unilateral spatial neglect. J. Clin. Exp. Neuropsychol 41(3), 270–289. [DOI] [PubMed] [Google Scholar]
- Wechsler D, 1997. Wechsler Memory Scale (WMS-III). Psychological Corporation, San Antonio, TX. [Google Scholar]
- Wilhelm B, Wilhelm H, Lüdtke H, 1999. Pupillography: Principles and applications in basic and clinical research, in: Kuhlmann J, Böttcher M (Eds.), Pupillography: Principles, Methods and Applications. Zuckschwerdt Verlag, München, pp. 1–10. [Google Scholar]
- Wilson RS, Nag S, Boyle PA, Hizel LP, Yu L, Buchman AS, Schneider JA, Bennett DA, 2013. Neural reserve, neuronal density in the locus ceruleus, and cognitive decline. Neurology 80(13), 1202–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.