Abstract
Brugada phenocopies represent some unusual clinical cases with identical characteristics to Brugada syndrome (BrS) elicited by various clinical circumstances. We report the case of a woman exhibiting “Brugada Phenocopy” during an acute anterior myocardial infarction, highlighting differential diagnosis with true BrS and discussing possible mechanisms underlying its dynamic ECG pattern.
Keywords: phenocopy, Brugada syndrome, ischemia, acute myocardial infarction
True congenital Brugada syndrome (BrS) is a primary electrical condition characterized by right precordial ST‐elevation predisposing to malignant ventricular arrhythmias and sudden cardiac death in patients with apparent structurally normal hearts.1 The term “Brugada Phenocopy” (BrP) is a relatively new terminology that has been applied to some unusual cases presenting identical ECG pattern to BrS but elicited by various clinical circumstances.2, 3 This term should be used to replace “Brugada‐like” patterns in the absence of BrS in order to achieve consistency in scientific literature.
CLINICAL CASE
A 49‐year‐old woman presented to the emergency department (ED) with intermittent chest pain over the last 48 hours. Five hours prior to admission, she presented continuous chest pain at rest resolving few minutes after starting a nitroglycerin infusion in the ED. Her baseline ECG showed sinus rhythm at 65 bpm, coved‐type ST‐segment elevation in leads V1–V3, and saddle‐back morphology in lead V4 (Fig. 1, panel A) evolving to ongoing transmural myocardial injury pattern during the next hour (Fig. 1, panels B and C); BP: 150/90 mmHg, normal cardiovascular examination, lower lung crackles; serum troponin I: 106.8 ng/mL (normal values < 0.40 ng/mL). The diagnosis of acute anterior myocardial infarction was confirmed and the patient was immediately referred for primary angioplasty. Coronary angiography showed an occlusion in the mid‐left anterior descending coronary artery. Successful primary angioplasty followed by implantation of two drug‐eluting stents was performed (Fig. 2, panels A1–A3). ST‐segment morphology evolution after percutaneous intervention is shown in Figure 2 (panel B).
Figure 1.
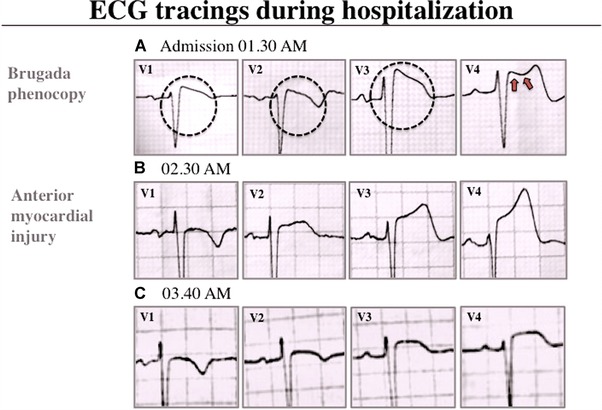
Surface ECG during hospitalization. (A) ECG obtained on admission (5 hours after onset of chest pain
Figure 2.
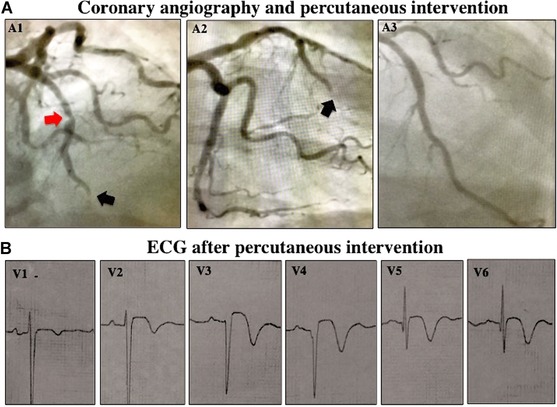
Coronary angiography and ECG after percutaneous angioplasty. (A) Coronary angiogram in cranial left anterior and right anterior oblique views (panels A1 and A2). Red and black arrows indicate severe stenosis of mid‐left anterior descending artery and distal occlusive thrombosis, respectively. (B) ECG after percutaneous coronary intervention.
DISCUSSION
Conditions inducing BrP can be characterized into six etiologic categories: metabolic disorders, mechanical compression, pulmonary embolism and myocardial ischemia, myocardial and pericardial diseases, ECG modulations, and miscellaneous.4, 5 A systematic approach to diagnose BrP is very useful to differentiate between this entity and BrS (Table 1).5
Table 1.
Current Criteria to Diagnose BrP
| Number | Diagnosis Criteria | Observed Result |
|---|---|---|
| I | Baseline ECG pattern | Type‐1 or type‐2 Brugada morphology |
| II | Identifiable underlying condition | Yes |
| III | Evolution of ECG pattern | Resolves upon resolution of underlying condition |
| IV | Clinical pretest probability of true BrS | Low |
| V | Provocative testing with sodium channel blockera | Negative |
| VI | Genetic testingb | Negative |
I–V criteria are mandatory for BrP.5
aAjmaline, flecainide, or procainamide. Not mandatory if surgical right ventricular outflow tract manipulation has occurred within last 96 hours; bDesirable but not mandatory.
This remains an important task to be resolved since ischemia may either induce a BrP or, inversely, unmask a true congenital BrS.4 In this clinical situation, current criteria allowed us to confirm the diagnosis of Type‐1, Class B BrP.5, 6, 7 Although the case presented here would require a challenge with a sodium channel blocker (ajmaline, flecainide, or procainamide), we did not perform these tests given the severe life‐threatening clinical situation and complete reversal of the ECG pattern once myocardial ischemia resolved. Genetic testing, although desirable is not necessary in all situations, since the SCN5A mutation is identifiable in only 20–30% of probands affected by true BrS.8
Recent availability of an International Registry and Educational Portal on BrP (http://www.brugadaphenocopy.com) as an expanding prospective registry aimed to determine incidence and importance of such patients represents a valuable resource to document different clinical presentations and stimulate further research.4, 5 Few prior clinical descriptions of BrP observed during acute myocardial ischemia can be found in the literature and properly). summarized in the International Registry.4, 5
A reduction in transient fast inward sodium current, the disorder most frequently observed in BrS mutations, produces an imbalance between input and output of positive charges at the end of phase 1 of the action potential. These altered ionic currents favor the development of characteristic notch and loss of the dome in action potential, which are due to altered transient outward potassium current (Ito). As density of Ito is greater in epicardium than in endocardium, this phenomenon occurs heterogeneously through the ventricular wall. If changes in notch are sufficiently marked, the epicardium action potential is significantly lengthened compared to the endocardium and ST‐segment elevation and negative T‐wave can be manifested on surface ECG.9
Although research on cellular basis of true BrS is constantly evolving, physiological mechanisms underlying BrP in different conditions such as acute myocardial ischemia are still poorly understood. Di Diego et al. analyzed potential physiological similarities between ECG patterns observed in an experimental BrS model and an acute transmural no‐flow ischemia model using isolated canine right ventricle wedge preparations. The researchers recorded transmembrane action potentials from endocardium and epicardium in both experimental models.10 In the presence of a prominent Ito‐mediated action potential notch, they found that no‐flow ischemia causes true ST‐segment elevation because of selective depression and loss of action potential dome at some epicardial sites. Conversely, in the absence of a prominent action potential notch, ischemia produced an apparent ST‐segment elevation due to marked transmural conduction delays. BrS model generated in preparations displaying a large epicardial Ito demonstrated that ST‐segment elevation was produced by loss of epicardial action potential dome only at some sites. These results highlighted that Ito could modulate ECG manifestations of acute ischemia as well as that of BrS, suggesting both clinical entities could be the result of a similar electrophysiological substrate.
In conclusion, recognition of BrP as the main differential diagnosis of true BrS is of paramount importance, in order to avoid unnecessary tests and interventions. The prognosis of BrP seems to be related to the evolution of the underlying condition; however, the natural history of BrP is not completely understood and is expected to emerge from the follow‐up of the International Registry.
Conflicts of interest: None.
REFERENCES
- 1. Bayes de Luna A, Brugada J, Baranchuk A, et al. Current electrocardiographic criteria for diagnosis of Brugada pattern: A consensus report. J Electrocardiol 2012;45:433–442. [DOI] [PubMed] [Google Scholar]
- 2. Baranchuk A, Nguyen T, Ryu MH, et al. Brugada phenocopy: New terminology and proposed classification. Ann Noninvasive Electrocardiol 2012;17:299–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anselm DD, Baranchuk A. Brugada phenocopy: Redefinition and updated classification. Am J Cardiol 2013;111:453. [DOI] [PubMed] [Google Scholar]
- 4. Gottschalk BH, Anselm DD, Baranchuk A. Brugada phenocopy international registry and online educational portal. Available at: http://www.brugadaphenocopy.com. Accessed November 6, 2015.
- 5. Anselm DD, Gottschalk B, Baranchuk A. Brugada phenocopies: Consideration of morphologic criteria and early findings from an International Registry. Can J Cardiol 2014;30:1511–1515. [DOI] [PubMed] [Google Scholar]
- 6. Gottschalk BH, Anselm DD, Baranchuk A. Coronary anomalies resulting in ischemia induced Brugada phenocopy. Int J Cardiol 2015;199:75–76. [DOI] [PubMed] [Google Scholar]
- 7. Gottschalk B, Anselm DD, Baranchuk A. Brugada phenocopy: Morphological classification and importance of provocative testing. Ann Noninvasive Electrocardiol 2014;19(6):604–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gottschalk BH, Anselm DD, Baranchuk A. Brugada phenocopy indecued by ischemia or Brugada syndrome unmasked by ischemia? Int J Cardiol 2014;177(2):619–620. [DOI] [PubMed] [Google Scholar]
- 9. Antzelevitch C, Pollevick GD, Cordeiro JM, et al. Loss‐of‐function mutations in the cardiac calcium channel underlie a new clinical entity characterized by ST‐segment elevation, short QT intervals, and sudden cardiac death. Circulation 2007;115:442–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Di Diego JM, Fish JM, Antzelevitch C. Brugada syndrome and ischemia‐induced ST‐segment elevation. Similarities and differences. J Electrocardiol 2005;38(Suppl. 4):14–17. [DOI] [PMC free article] [PubMed] [Google Scholar]


