Abstract
Background: Autonomic markers, such as heart rate variability (HRV), heart rate turbulence (HRT), and baroreflex sensitivity (BRS) provide information on the risk of all‐cause mortality after an acute myocardial infarction (AMI), but their value in predicting nonfatal cardiac events is not well known.
Methods: A consecutive series of 675 patients with an AMI were followed up to 30 months. At baseline, the patients underwent a 24‐hour Holter recording, and assessment of BRS using phenylephrine test. Several parameters of HRV and HRT were determined.
Results: After the follow‐up, 98 patients (15%) had a nonfatal acute coronary event. Among the studied variables, the short‐term scaling exponent alpha1 (P = 0.002), power‐law slope beta (P = 0.008), low‐frequency component of HRV power spectrum (P < 0.001), turbulence slope (P < 0.001), and BRS (P < 0.001) had the strongest association with the occurrence of nonfatal acute coronary events in univariate comparisons. After adjustment with relevant clinical variables (such as age, gender, ejection fraction, functional class, medication, diabetes) in the Cox proportional hazards model, alpha1 and beta remained as statistically significant predictors of nonfatal acute coronary events (HR = 2.0 [1.2–3.2, 95% CIs, P = 0.006] for alpha1 ≤ 1.025), (HR = 1.9 [1.2–3.1, P = 0.008] for beta ≤–1.507).
Conclusion: Several autonomic markers provide information on the risk of recurrent nonfatal coronary events after an AMI. Altered fractal heart rate behavior seems to be the strongest independent predictor of such events.
Keywords: heart rate, heart rate variability, heart rate turbulence, baroreflex sensitivity, myocardial infarction
Several autonomic markers, such as heart rate variability (HRV), heart rate turbulence (HRT), and baroreflex sensitivity (BRS), predict mortality in postinfarction patients. 1 , 2 , 3 However, data about their value in predicting nonfatal cardiac events after an acute myocardial infarction (AMI) are limited. 4 Unlike sudden cardiac deaths, 5 , 6 recurrent coronary events in general postinfarction populations are not rare in the current treatment era. It would be important to recognize the patients who are at the highest risk for recurrent acute coronary events. These patients could be selected for most advanced invasive and drug treatment strategies including sustained effective antithrombotic, aggressive lipid lowering, and long‐acting angiotensin converting enzyme‐inhibiting therapies. Therefore, this study was aimed to assess the value of autonomic markers (HRV, HRT, and BRS) in predicting the risk of recurrent acute coronary events during a follow‐up of a consecutive series of patients with an AMI.
METHODS
Study Population
A single‐center, prospective follow‐up study, the Multiple Risk Factor Analysis Trial (MRFAT), 7 , 8 included a consecutive series of patients with AMI during the first 7 days after the initial event. The details of the study have been published previously. 7 , 8 The diagnosis of AMI was confirmed according to modern guidelines. 8 The patients were excluded, if they were older than 75 years, had unstable angina at recruitment, dementia, alcoholism, drug abuse, nonsinus rhythm, or were unable to give an informed consent. If a patient underwent coronary bypass operation before discharge or died during the hospital stay, he/she was not included in the analysis. The Ethical Committee of the University of Oulu approved the study protocol, and all patients were required to give an informed consent. A special emphasis was put on optimizing cardiac medication, particularly beta‐blocker therapy, by the time of discharge. The study initially included 700 patients with AMI.
Left Ventricular Function
Left ventricular systolic function was measured with two‐dimensional echocardiography from 2 to 7 days after AMI using methodology that has been described in detail previously. 8
Electrocardiographic Recordings
The patients had a 24‐hour ECG recording between days 5 and 14 after AMI using an Oxford Medilog system (Oxford Medilog 4500, Oxford Medical Ltd., Oxford, United Kingdom).
Analyses of Autonomic Markers
The standard deviation of all NN intervals (SDNN) measured from the 24‐hour recording, and low‐frequency (LF) and high‐frequency (HF) components of the HRV power spectrum using standard techniques, 9 were determined as conventional measurements of HRV. The detrended fluctuation analysis was used to calculate the short‐term scaling exponent α1. In this technique, the variability of integrated and detrended time series is measured in observation windows of different sizes and shown as a function of the size of the observation window on a log‐log scale. The short‐term scaling exponent α1 describes the short‐term (<11 beats) fractal‐like scaling properties of the RR interval time series. The details of the method have been published previously. 10 , 11 , 12 For estimating the long‐term fractal heart rate behavior, the power‐law slope β was determined. A plot of spectral power and frequency on a log‐log scale shows linear portion between 10−4 and 10−2 Hz. The slope of this relationship describes long‐term scaling characteristics of HRV in the region of the ultralow‐ and very‐low‐frequency bands. The details of the method are described elsewhere. 13 , 14 HRV was analyzed from 89% of the follow‐up patients.
Turbulence slope (TS) was analyzed as a measure of HRT. TS was calculated as the highest slope of the regression line over any of the five successive sinus beat RR intervals during first 15 sinus beat RR intervals after a ventricular premature depolarization. The analysis was done from the averaged RR intervals following the ventricular premature depolarizations. The details of the method have been published previously. 2 , 15 HRT could be analyzed from 83% of the follow‐up patients.
BRS was measured as the rate‐pressure response to intravenous phenylephrine using the methodology described in detail elsewhere. 16 The analysis was done between the days 5 and 21 after the AMI for 69% of the follow‐up patients.
Follow‐Up and End Points
For the occurrence of nonfatal acute coronary events, the patients were followed up to 30 months after the AMI. A nonfatal acute coronary event was defined as a recurrent acute myocardial infarction or unstable angina pectoris, which did not result in death. A recurrent myocardial infarction was confirmed according to same guidelines that were used for the initial event. 8 The first recurrent nonfatal acute coronary event during the follow‐up period was considered as a primary endpoint of this analysis. Total mortality was also analyzed during the follow‐up period for comparing the predictive power of the autonomic markers between nonfatal acute coronary events and all‐cause mortality as endpoints.
Statistical Analysis
The SPSS statistical software (version 11.5, SPSS Inc., Chicago, IL, USA) was used in the analysis of the data. Statistical significances of differences in continuous clinical variables and autonomic markers between patients with and without recurrent acute coronary events during the follow‐up were assessed using the standard t‐test. The chi‐square test was used for studying the statistical significances of the differences in categorical variables between the patients with and without the primary endpoint. The receiver operator characteristics (ROC) curve analysis was used to evaluate the accuracy of the autonomic markers in predicting recurrent coronary events, and to determine optimal cut points of these markers at the sensitivity range from 25% to 50%. The Kaplan‐Meier curves were computed to show cumulative proportional probabilities of recurrent nonfatal acute coronary events and all‐cause mortality in patients with and without unfavorable values of autonomic risk markers. The log rank test was used to assess the statistical significance of the differences between the curves. The multivariate Cox regression analysis was used to adjust the autonomic risk markers to age and gender and other risk variables. The correlations between the autonomic risk markers were estimated by analyzing the Pearson correlation coefficients. A P value < 0.05 was considered to be statistically significant.
RESULTS
Of the 700 patients, 675 were discharged alive. After these 675 patients were followed up to 30 months, 98 (15%) patients had experienced a nonfatal acute coronary event (20 ± 12 months follow‐up on average), and 68 (10%) had died (28 ± 6 months follow‐up on average). The clinical characteristics of the patients with and without a recurrent coronary event during the follow‐up are shown in Table 1. The patients who had an acute coronary event during follow‐up, were significantly older, more often of female sex, had significantly worse functional class, and more frequently having diabetes than patients without such an event. Patients with a recurrent coronary event were statistically significantly more frequently on warfarin, digitalis, and calcium‐blocker therapy compared with the patients without a recurrent coronary event.
Table 1.
Clinical Characteristics of the Study Patients with and without a Recurrent Acute Coronary Event during the Follow‐Up
| Without Recurrent Coronary Event (n = 577) | With Recurrent Coronary Event (n = 98) | P | |
|---|---|---|---|
| Age (years) | 61 ± 10 | 66 ± 9 | <0.001 |
| Male/female | 439/138 (76%/24%) | 64/34 (65%/35%) | 0.02 |
| Ejection fraction (%) | 45 ± 9 | 44 ± 9 | 0.10 |
| NYHA class, I/II/III | 411/107/59 (71%/19%/10%) | 55/21/22 (56%/21%/23%) | <0.01 |
| History | |||
| Diabetes | 123 (21%) | 36 (37%) | <0.01 |
| Previous AMI | 118 (20%) | 28 (29%) | 0.07 |
| Hypertension | 280 (49%) | 53 (54%) | 0.32 |
| Smoking | 385 (67%) | 71 (72%) | 0.26 |
| Type of AMI, Q/non‐Q/Int | 291/245/41 (50%/43%/7%) | 41/50/7 (42%/51%/7%) | 0.42 |
| Location of AMI, Ant/Inf/Int | 257/249/71 (45%/43%/12%) | 50/33/15 (51%/34%/15%) | 0.08 |
| Medication | |||
| β‐blockers | 559 (97%) | 92 (94%) | 0.06 |
| Statins | 200 (35%) | 36 (37%) | 0.72 |
| Aspirin | 483 (84%) | 75 (77%) | 0.06 |
| Warfarin | 60 (10%) | 17 (17%) | 0.04 |
| ACE/ATII‐inhibitors | 217 (38%) | 46 (47%) | 0.09 |
| Diuretic drugs | 138 (24%) | 32 (33%) | 0.07 |
| Digitalis | 31 (5%) | 12 (12%) | <0.05 |
| Ca‐blockers | 37 (6%) | 16 (16%) | <0.01 |
| Amiodarone | 9 (2%) | 3 (3%) | 0.30 |
The values are means ± SD or the number of the patients. ACE = angiotensin‐converting enzyme; AMI = acute myocardial infarction; ATII = angiotensin II; Ca = calcium; NYHA = New York Heart Association; Q/non‐Q/Int = Q‐wave/non‐Q‐wave/Indeterminate.
Autonomic Markers as Predictors of Recurrent Acute Coronary Events
The values of LF, the power‐law slope β, the short‐term scaling exponent α1, TS, and BRS were statistically significantly lower in patients with a recurrent acute coronary event compared to the patients without such an event during the follow‐up (Table 2). All these autonomic markers, except LF, also showed significant association with the occurrence of recurrent coronary events in a univariate Cox regression analysis (Table 3). After adjustment with relevant clinical variables, such as age, gender, ejection fraction, functional class, diabetes, and medication, in the Cox proportional hazards model, the short‐term scaling exponent α1 and the power‐law slope β remained as statistically significant predictors of nonfatal acute coronary events (Table 3).
Table 2.
Autonomic Markers in Study Patients
| Without Recurrent Coronary Event (n = 577) | With Recurrent Coronary Event (n = 98) | P | |
|---|---|---|---|
| Heart rate (Holter‐based) | 57.8 ± 10.4 | 57.3 ± 11.9 | 0.65 |
| HRV | |||
| SDNN (ms) | 97 ± 32 | 91 ± 35 | 0.14 |
| HF (ms2) | 258 ± 541 | 193 ± 212 | 0.053 |
| LF (ms2) | 474 ± 627 | 305 ± 332 | <0.001 |
| Power‐law slope β | −1.30 ± 0.19 | −1.38 ± 0.24 | 0.008 |
| Short‐term scaling exponent α1 | 1.23 ± 0.23 | 1.12 ± 0.33 | 0.002 |
| HRT | |||
| Turbulence slope (ms/NN) | 5.65 ± 6.09 | 3.81 ± 3.91 | <0.001 |
| BRS | |||
| Rate‐pressure response (ms/mmHg) | 9.38 ± 8.45 | 6.20 ± 5.27 | <0.001 |
The values are means ± SD. BRS = baroreflex sensitivity; HF = high‐frequency component of heart rate variability (HRV) power spectrum; HRT = heart rate turbulence; LF = low‐frequency component of HRV power spectrum; NN = normal‐to‐normal RR interval; SDNN = the standard deviation of all NN intervals. Look in the Methods section for details.
Table 3.
Autonomic Markers as Univariate and Multivariate Predictors of Recurrent Coronary Events
| Autonomic Markers | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| LF ≤ 139 ms2 | 1.38 | (0.88–2.14) | 0.16 | 0.88 | (0.55–1.42) | 0.60 |
| Power‐law slope β≤−1.507 | 2.45 | (1.54–3.89) | <0.001 | 1.92 | (1.19–3.12) | 0.008 |
| Short‐term scaling exponent α1≤ 1.025 | 3.04 | (1.96–4.73) | <0.001 | 1.98 | (1.21–3.22) | 0.006 |
| Turbulence slope ≤ 1.3 ms/NN | 1.67 | (1.05–2.66) | 0.03 | 1.24 | (0.77–2.00) | 0.39 |
| BRS ≤ 2.63 ms/mmHg | 2.21 | (1.25–3.90) | 0.007 | 1.34 | (0.72–2.50) | 0.35 |
HR = Hazards ratios obtained from the Cox regression. CI = confidence intervals. Other abbreviations are same as in Table 2.
Accuracy of Autonomic Markers in Predicting Nonfatal Coronary Events
When optimal cutpoints were defined from the ROC curves at the sensitivity level from 25% to 50% for the Kaplan‐Meier curve analysis, α1, β, TS, and BRS, all had a statistically significant power in discriminating the patients with and without recurrent coronary events during the follow‐up (1, 2). The fractal measure of HRV, the short‐term scaling exponent α1 in particular, performed the best in separating the patients with and without acute coronary events at this sensitivity level. In the ROC curve analysis, all the four above‐mentioned autonomic markers had a similar overall accuracy in predicting recurrent acute coronary events (3, 4). However, the fractal measures of HRV, the short‐term scaling exponent α1 in particular, performed somewhat better at high specificity, and TS and BRS at high sensitivity levels. The overall accuracy of the fractal measures of HRV and TS in predicting all‐cause mortality was somewhat better than in predicting recurrent coronary events (3, 4). However, at the optimal cutpoint for coronary events in the sensitivity range from 25% to 50%, the predictive accuracy of the short‐term scaling exponent for acute coronary events and all‐cause mortality was similar (1, 2).
Figure 1.
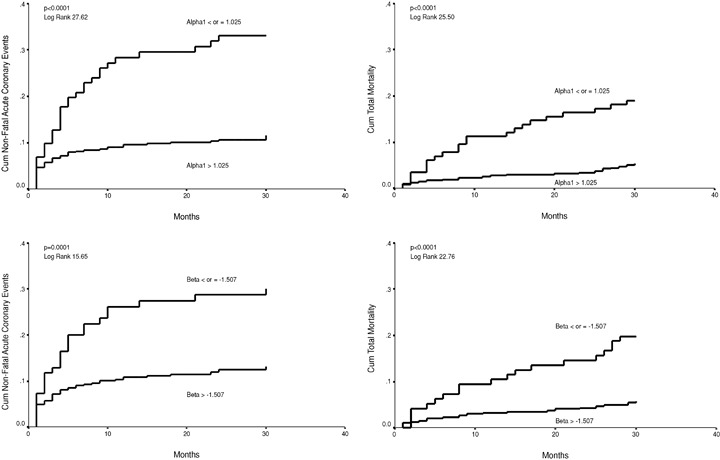
Cumulative proportional probability of nonfatal acute coronary events for patients with the short‐term scaling exponent alpha1 ≤ or > 1.025 (upper left chart), for patients with the power‐law slope beta ≤ or > –1.507 (lower left chart). Cumulative proportional probability of all‐cause mortality for patients with the short‐term scaling exponent alpha1 ≤ or > 1.025 (upper right chart), for patients with the power‐law slope beta ≤ or > –1.507 (lower right chart).
Figure 2.
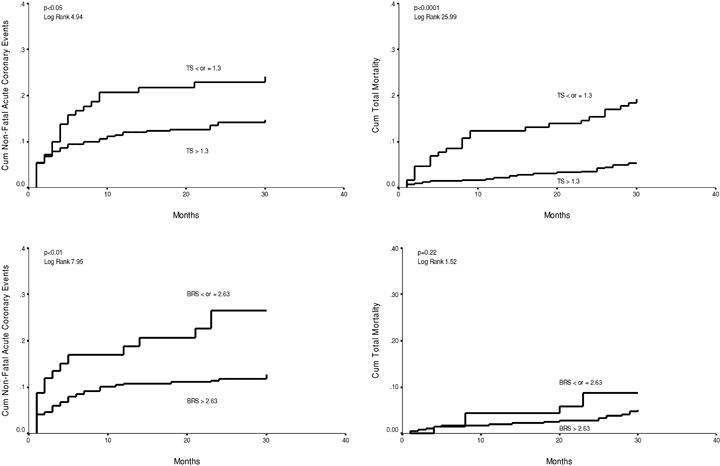
Cumulative proportional probability of nonfatal acute coronary events for patients with the turbulence slope (TS) ≤ or > 1.3 ms/NN (upper left chart), for patients with the baroreflex sensitivity (BRS) ≤ or > 2.63 ms/mmHg (lower left chart). Cumulative proportional probability of all‐cause mortality for patients with the turbulence slope (TS) ≤ or > 1.3 ms/NN (upper right chart), for patients with the BRS ≤ or > 2.63 ms/mmHg (lower right chart).
Figure 3.
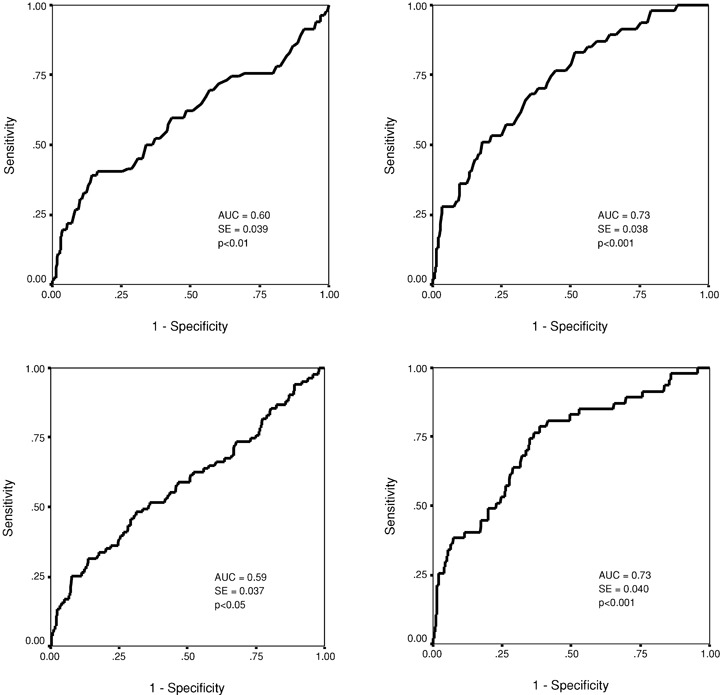
Receiver operator characteristics (ROC) curve for the short‐term scaling exponent α1 in predicting nonfatal acute coronary events (upper left chart) and all‐cause mortality (upper right chart). The ROC curve for the power‐law slope β in predicting nonfatal acute coronary events (lower left chart) and all‐cause mortality (lower right chart). AUC = area under the curve; SE = standard error.
Figure 4.
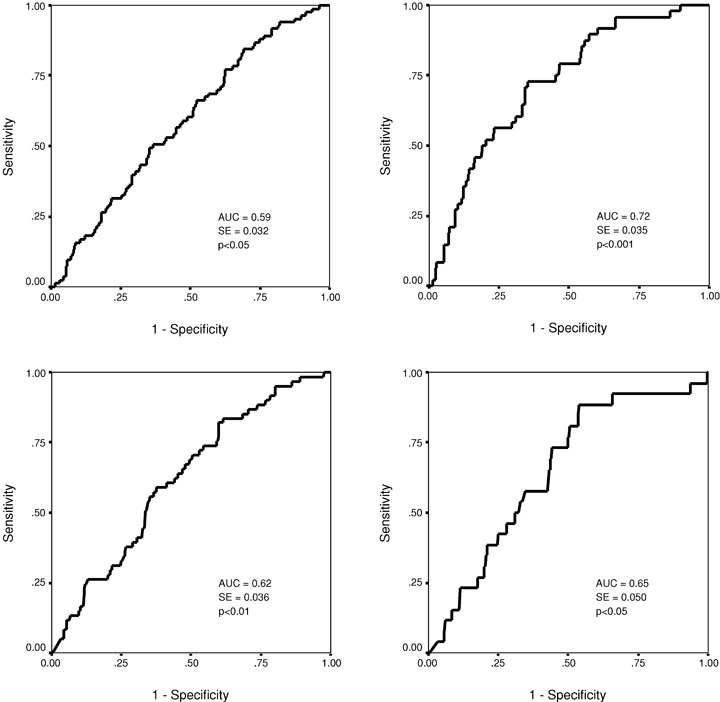
Receiver operator characteristics (ROC) curve for the turbulence slope in predicting nonfatal acute coronary events (upper left chart) and all‐cause mortality (upper right chart). The ROC curve for the baroreflex sensitivity in predicting nonfatal acute coronary events (lower left chart) and all‐cause mortality (lower right chart). AUC = area under the curve; SE = standard error.
Associations between the Autonomic Markers
The correlations between the fractal measures of HRV, LF, TS, and BRS are shown in Table 4. All these autonomic markers had a significant, but relatively weak correlation with each other. The short‐term scaling exponent α1 and the power‐law slope β had somewhat closer relationship to each other, and LF, TS, and BRS to each other than to the other autonomic markers.
Table 4.
Associations of the Autonomic Markers with Each Other in the Study Population
| LF | β | α1 | TS | BRS | |
|---|---|---|---|---|---|
| LF | 0.27† | 0.11* | 0.38† | 0.44† | |
| β | 0.27† | 0.36† | 0.30† | 0.24† | |
| α1 | 0.11* | 0.36† | 0.23† | 0.17† | |
| TS | 0.38† | 0.30† | 0.23† | 0.34† | |
| BRS | 0.44† | 0.24† | 0.17† | 0.34† |
The values are Pearson's correlation coefficients. α1= the short‐term scaling exponent, β= the power‐law slope; BRS = baroreflex sensitivity; LF = the low‐frequency component of heart rate variability power spectrum; TS = turbulence slope. *= P < 0.05, †= P < 0.001.
DISCUSSION
This analysis shows that several autonomic markers, including the short‐term and long‐term fractal measures of HRV, HRT, and BRS, predicted the occurrence of nonfatal recurrent acute coronary events during the follow‐up of consecutive series of survivors with AMI of whom the vast majority was on beta‐blocking medication. The short‐term scaling exponent α1, and the power‐law slope β, retained their predicting power after adjusting with relevant clinical variables suggesting that the altered fractal heart rate behavior yields important independent information about the risk of nonfatal acute coronary events in postinfarction patients.
Heart Rate Variability and Cardiac Events
Several studies have shown that impaired HRV predicts mortality in postinfarction patients. 17 , 18 , 19 However, the value of HRV parameters in predicting nonfatal cardiac events is not well known. Tsuji et al. evaluated the impact of reduced HRV on risk for cardiac events in a community‐based population free of clinically apparent coronary artery disease or congestive heart failure. 4 They used conventional measures of HRV and found that decreased HRV was significantly associated with the risk for a cardiac event, which was defined as angina pectoris, myocardial infarction, coronary heart disease death, or congestive heart failure. Their findings can be partly explained by the notion that decreased HRV may reflect subclinical cardiac disease. Our follow‐up study included patients with AMI. Therefore our findings cannot be attributed to the detection of occult coronary artery disease by reduced HRV. However, the analysis of HRV can give an insight into the progression of focal coronary atherosclerosis, 20 a phenomenon that may be a mechanistic link between the association of impaired HRV and increased occurrence of recurrent coronary events in the present patients with AMI.
Among the studied autonomic markers, only the short‐term scaling exponent α1 and the power‐law slope β, remained statistically significant predictors of nonfatal acute coronary events after adjusting with clinical variables. This finding supports the concept that altered fractal heart rate behavior may reveal more delicate pathophysiological disturbances in relation to the risk for recurrent coronary events than can be detected by conventional measures of HRV, HRT, or BRS. Recent experiments in healthy subjects show that sympathetic activation in the presence of enhanced vagal outflow results in decreased short‐term fractal organization of heart rate dynamics represented by lower values of the short‐term scaling exponent α1. 21 This observation supports the view that short‐term fractal heart rate behavior is determined by sympathovagal interaction also in pathological conditions, such as myocardial infarction. The short‐term scaling exponent has a relatively good correlation with the LF/HF ratio in controlled recording conditions, but the association is weak in ambulatory 24‐hour recording conditions. 22 In patients with extensive myocardial infarction, LF is usually reduced and HF relatively preserved or only slightly decreased resulting also in a decreased short‐term scaling exponent. 23 In this study, the patients who experienced a nonfatal coronary event during the follow up had significantly lower LF compared to the patients without such an event. This may indicate more extensive myocardial infarction/more advanced coronary heart disease in patients who had a recurrent coronary event.
The mechanism why autonomic markers predict mortality after AMI has not been clear. Most often, altered autonomic regulation has been considered to be associated with vulnerability to fatal arrhythmias. 1 This study provides further insight into the possible mechanistic link. It seems plausible to speculate that altered autonomic regulation increases the risk of acute complications of atherosclerotic plaques, leading either to a nonfatal coronary event, sudden death, or even to progressive heart failure.
Heart Rate Turbulence and Baroreflex Sensitivity as Predictors of Cardiac Events
HRT is considered to be a measure of the autonomic response to perturbations of blood pressure after single ventricular premature depolarization and to be significantly associated with BRS. 2 , 15 , 24 In our study, there was a significant, but relatively weak correlation between HRT and BRS. Both HRT and BRS are known to predict mortality after AMI. 2 , 3 , 6 , 15 , 25 However, data on their value in predicting nonfatal coronary events are very limited. In this study, both HRT and BRS were significant predictors of recurrent acute coronary events in patients with AMI. HRT and BRS had a similar overall accuracy in predicting coronary events compared to the fractal HRV measures. However, the fractal measures of HRV, the short‐term scaling exponent in particular, performed better at high specificity levels, especially when the cut‐point was optimized. In addition, HRT and BRS did not remain as significant predictors of coronary events after adjusting with clinical variables. All the four above‐mentioned autonomic markers, except BRS, had somewhat better accuracy in predicting all‐cause mortality than in predicting coronary events. However, at the cutpoint optimized for coronary events, the predicting power of the short‐term scaling exponent was similar for nonfatal acute coronary events and all‐cause mortality.
Potential Clinical Implications and Study Limitations
High‐risk postinfarction patients with severely depressed left ventricular function benefit from a prophylactic implantable cardioverter‐defibrillator therapy. 26 Although the vast majority of sudden cardiac deaths occurs in patients with relatively well‐preserved left ventricular function, it is difficult to identify these patients in advance as the annual incidence of sudden cardiac death is under 1% in general AMI populations during the modern treatment era. 5 , 6 As shown in this study, nonfatal acute recurrent coronary events are much more common in patients with AMI. Identifying the patients at highest risk of recurrent coronary events for optimal treatments, would prevent some of these patients to shift toward more higher‐risk categories with need for device therapies. If the present findings can be confirmed in other follow‐up studies, the autonomic markers, the short‐term fractal heart rate behavior in particular, could serve as markers for the risk of recurrent acute coronary events in patient with AMI.
Autonomic markers may have different value in predicting cardiac events in patients with and without preserved left ventricular function. 6 Due to limited number of patients with depressed left ventricular function, we were not able to do subgroup analyses in the present AMI population. Somewhat smaller proportion of patients had BRS testing, which may cause a potential bias in terms of predictive accuracy of this parameter in this analysis.
CONCLUSION
Several autonomic markers predict nonfatal recurrent acute coronary events after AMI. Breakdown of heart rate fractal organization seems to be the most powerful independent predictor of such events.
Acknowledgments
Acknowledgments: This study was supported in part by grants from the Medical Council of the Finnish Academy of Science, Helsinki, Finland and the Sigrid Juselius Foundation, Helsinki, Finland.
REFERENCES
- 1. Huikuri HV, Mäkikallio T, Airaksinen KEJ, et al Measurement of heart rate variability: A clinical tool or a research toy? J Am Coll Cardiol 1999;34:1878–1883. [DOI] [PubMed] [Google Scholar]
- 2. Schmidt G, Malik M, Barthel P, et al Heart‐rate turbulence after ventricular premature beats as a predictor of mortality after acute myocardial infarction. Lancet 1999;353:1390–1396. [DOI] [PubMed] [Google Scholar]
- 3. La Rovere MT, Bigger JT Jr, Marcus FI, et al Baroreflex sensitivity and heart‐rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes after Myocardial Infarction) Investigators. Lancet 1998;351:478–484. [DOI] [PubMed] [Google Scholar]
- 4. Tsuji H, Larson MG, Venditti FJ Jr, et al Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation 1996;94:2850–2855. [DOI] [PubMed] [Google Scholar]
- 5. Jordaens L, Tavernier R. MIRRACLE Investigators . Determinants of sudden death after discharge from hospital for myocardial infarction in the thrombolytic era. Eur Heart J 2001;22:1214–1225. [DOI] [PubMed] [Google Scholar]
- 6. Mäkikallio TH, Barthel P, Schneider R, et al Prediction of sudden cardiac death after acute myocardial infarction: Role of Holter monitoring in the modern treatment era. Eur Heart J 2005;26:762–769. [DOI] [PubMed] [Google Scholar]
- 7. Huikuri HV, Tapanainen JM, Lindgren K, et al Prediction of sudden cardiac death after myocardial infarction in the beta‐blocking era. J Am Coll Cardiol 2003;42:652–658. [DOI] [PubMed] [Google Scholar]
- 8. Tapanainen JM, Still AM, Airaksinen KEJ, et al Prognostic significance of risk stratifiers of mortality, including T wave alternans, after acute myocardial infarction: Results of a prospective follow‐up study. J Cardiovasc Electrophysiol 2001;12:645–652. [DOI] [PubMed] [Google Scholar]
- 9. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology . Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation 1996;93:1043–1065. [PubMed] [Google Scholar]
- 10. Iyengar N, Peng CK, Morin R, et al Age‐related alterations in the fractal scaling of cardiac interbeat interval dynamics. Am J Physiol 1996;271:R1078–R1084. [DOI] [PubMed] [Google Scholar]
- 11. Peng CK, Havlin S, Stanley HE, et al Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. CHAOS 1995;1:82–87. [DOI] [PubMed] [Google Scholar]
- 12. Huikuri HV, Makikallio TH, Peng CK, et al Fractal correlation properties of R‐R interval dynamics and mortality in patients with depressed left ventricular function after an acute myocardial infarction. Circulation 2000;101:47–53. [DOI] [PubMed] [Google Scholar]
- 13. Saul JP, Albrecht P, Berger RD, et al Analysis of long term heart rate variability: Methods, 1/f scaling and implications. Comput Cardiol 1988;14:419–422. [PubMed] [Google Scholar]
- 14. Bigger JT Jr, Steinman RC, Rolnitzky LM, et al Power law behavior of RR‐interval variability in healthy middle‐aged persons, patients with recent acute myocardial infarction, and patients with heart transplants. Circulation 1996;93:2142–2151. [DOI] [PubMed] [Google Scholar]
- 15. Barthel P, Schneider R, Bauer A, et al Risk stratification after acute myocardial infarction by heart rate turbulence. Circulation 2003;108:1221–1226. [DOI] [PubMed] [Google Scholar]
- 16. Airaksinen KE, Tahvanainen KU, Eckberg DL, et al Arterial baroreflex impairment in patients during acute coronary occlusion. J Am Coll Cardiol 1998;32:1641–1647. [DOI] [PubMed] [Google Scholar]
- 17. Kleiger RE, Miller JP, Bigger JT Jr, et al Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol 1987;59:256–262. [DOI] [PubMed] [Google Scholar]
- 18. Zuanetti G, Neilson JM, Latini R, et al Prognostic significance of heart rate variability in post‐myocardial infarction patients in the fibrinolytic era. The GISSI‐2 results. Gruppo Italiano per lo Studio della Sopravvivenza nell' Infarto Miocardico. Circulation 1996;94:432–436. [DOI] [PubMed] [Google Scholar]
- 19. Bigger JT Jr, Fleiss JL, Steinman RC, et al Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation 1992;85:164–171. [DOI] [PubMed] [Google Scholar]
- 20. Huikuri HV, Jokinen V, Syvänne M, et al Heart rate variability and progression of coronary atherosclerosis. Arterioscler Thromb Vasc Biol 1999;19:1979–1985. [DOI] [PubMed] [Google Scholar]
- 21. Tulppo MP, Kiviniemi AM, Hautala AJ, et al Physiological background of the loss of fractal heart rate dynamics. Circulation 2005;112:314–319. [DOI] [PubMed] [Google Scholar]
- 22. Perkiömäki JS, Mäkikallio TH, Huikuri HV. Fractal and complexity measures of heart rate variability. Clin Exp Hypertens 2005;27:149–158. [PubMed] [Google Scholar]
- 23. Huikuri HV, Mäkikallio TH. Heart rate variability in ischemic heart disease. Auton Neurosci 2001;90:95–101. [DOI] [PubMed] [Google Scholar]
- 24. Watanabe MA, Marine JE, Sheldon R, et al Effects of ventricular premature stimulus coupling interval on blood pressure and heart rate turbulence. Circulation 2002;106:325–330. [DOI] [PubMed] [Google Scholar]
- 25. La Rovere MT, Pinna GD, Hohnloser SH, et al ATRAMI Investigators. Autonomic tone and reflexes after myocardial infarction. Baroreflex sensitivity and heart rate variability in the identification of patients at risk for life‐threatening arrhythmias: Implications for clinical trials. Circulation 2001;103:2072–2077. [DOI] [PubMed] [Google Scholar]
- 26. Moss AJ, Zareba W, Hall WJ, et al Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346:877–883. [DOI] [PubMed] [Google Scholar]


