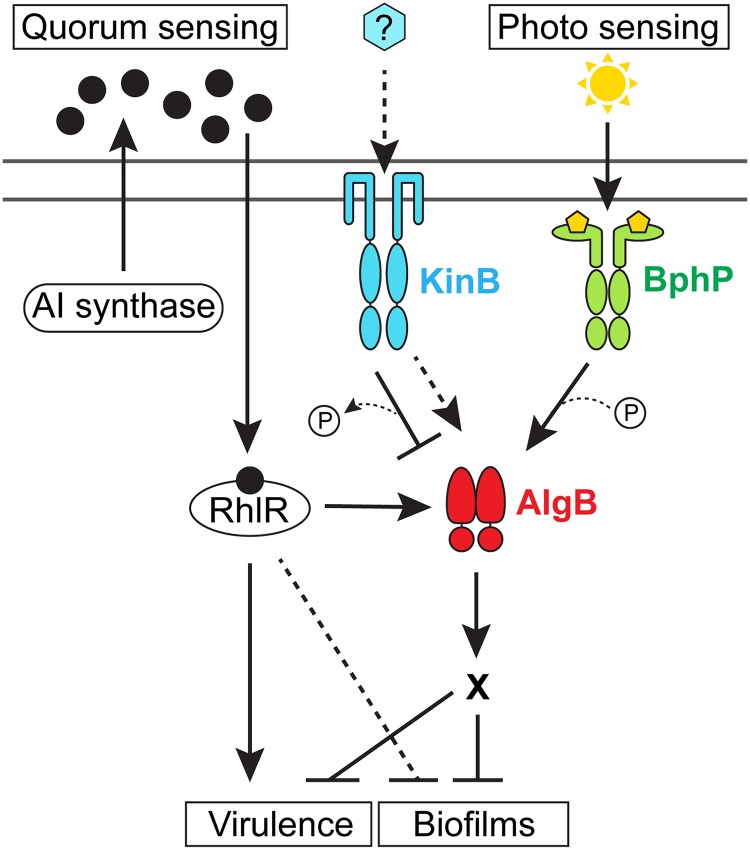
Fig 1. Model for P. aeruginosa integration of quorum-sensing and photosensing information into the control of virulence and biofilm development.
The RhlR quorum-sensing receptor binds its partner AI produced by either the RhlI or PqsE autoinducer synthase (black circles) at high cell density [22]. The RhlR–AI complex represses biofilm formation and virulence gene expression by activating transcription of the algB–kinB operon encoding the KinB HK and the AlgB RR, the latter an indirect repressor of biofilm formation. KinB antagonizes AlgB by dephosphorylation. The stimulus (blue hexagon) for KinB is unknown. In the presence of this putative stimulus, the hypothesis is that KinB functions as a kinase for AlgB (dashed arrow). Photosensing stimulates the BphP HK to autophosphorylate and subsequently transfer the phosphoryl group to AlgB to activate AlgB. AlgB-P activates transcription of genes required for repression of group behaviors such as biofilm formation and virulence. A “P” in a circle denotes addition or removal of a phosphate moiety (dotted line). X denotes that the genes functioning downstream of AlgB in the process are not known. The RhlR–AI complex directly activates virulence gene expression and also represses biofilm formation by additional unknown mechanisms (dashed T-bar). AI, autoinducer; HK, histidine kinase; RR, response regulator.