Figure 1.
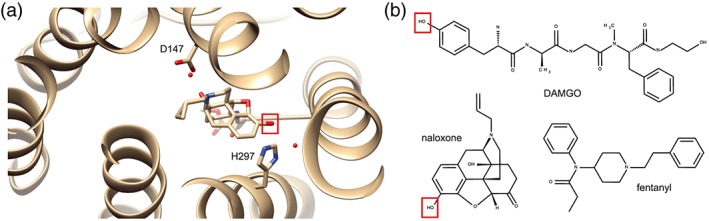
Structural concepts for the investigation of ligand‐specific pH‐sensitivity of binding to MOR. (a) Structure of the human μ‐opioid receptor bound to the antagonist β‐funaltrexamine (β‐FNA) modified from the PDB entry 4DKL (Manglik et al., 2012) with the software USCF Chimera (Pettersen et al., 2004). Histidine 2976.52 (H2976.52) forms a water‐mediated hydrogen bond to the hydroxyl group at C27 (red box) of β‐FNA. (b) Skeletal formulas of [D‐Ala2,N‐Me‐Phe4,Gly5‐ol]‐enkephalin (DAMGO), naloxone (NLX), and fentanyl; hydroxyl groups demonstrated (in case of DAMGO) or likely (in case of naloxone) to interact with μ‐opioid receptor residue H2976.52 are highlighted by red boxes
