Table 1.
Trypanothione and glutathione are the major, low MW thiols utilized by T. cruzi to keep redox homeostasis.
| Glutathione | Trypanothione | |
|---|---|---|
| A. Chemical structure Oxidized state: |
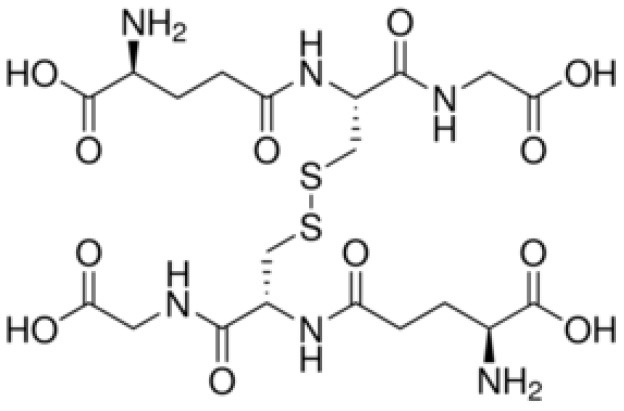 |
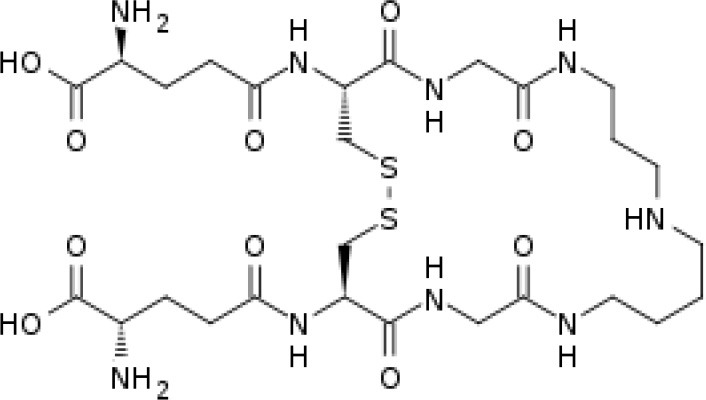 Número de envío [A |
| Reduced state: | 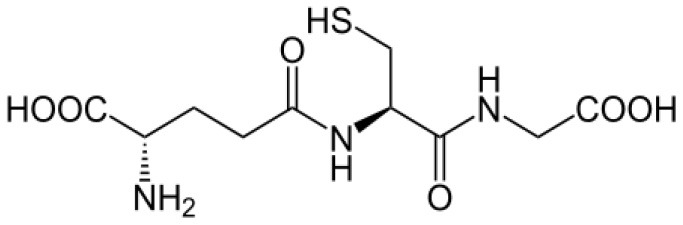 |
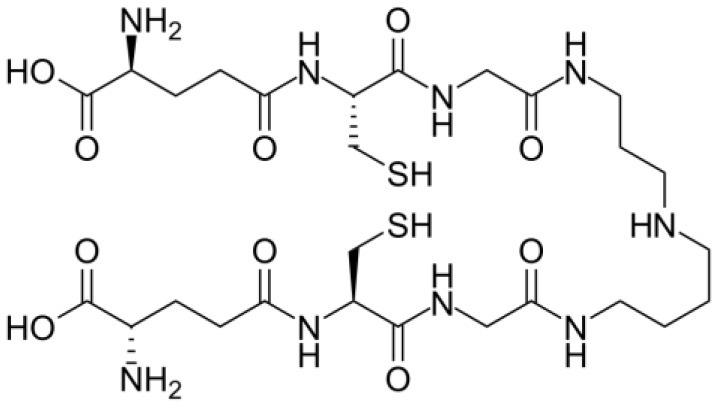 3552373] |
| B. Enzymes involved | GshA and GshB | TryS |
| C. Substrates | ATP L-Glutamate L-Cysteine L-Glycine |
ATP Spermidine GSH (*possibly synthesized by parasite) |
| D. Reduction coefficient (Fairlamb and Cerami, 1992) | Eo = −0.230 | Eo = −0.242 V |
| E. pKa (Moutiez et al., 1994) | 8.7 | 7.4 |
| F. Concentration | 1 mM in blood (Richie et al., 1996). 6.9 mM in intracellular milieu of HeLa cells (Montero et al., 2013) |
0.12–0.64 nmol/108 epimastigotes; 0.25–0.95 nmol/108 bloodstream trypomastigotes; 0.12 nmol/108 amastigotes (Ariyanayagam and Fairlamb, 2001; Ariyanayagam et al., 2003) |
Glutathione (ubiquitous monothiol among eukaryotes) and trypanothione (a kinetoplastid-specific dithiol) are synthesized by parasites' antioxidant machinery, and these molecules play a key role in distribution of reducing equivalents along the antioxidant network. Even when they have similar characteristics, T[SH]2 is considered a kinetically superior antioxidant.
