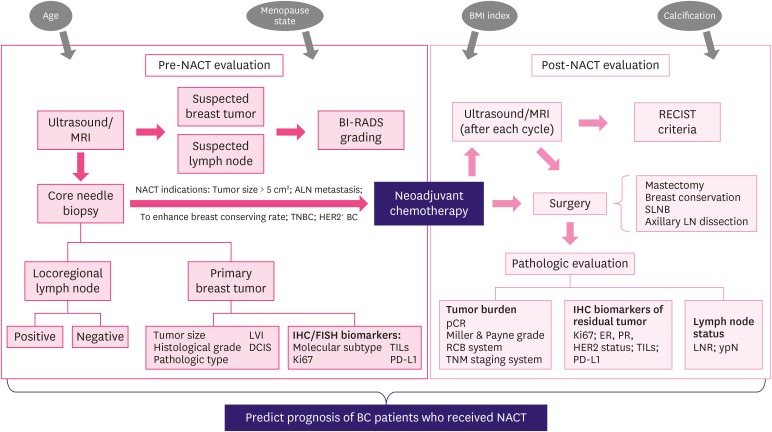
Figure 1. The general clinical evaluating process of BC patients before and after NACT. Some evaluating results might provide additional prognostic information for patients received NACT (TILs and PD-L1 examination have not been routinely used in current clinical practice).
BC = breast cancer; NACT = neoadjuvant chemotherapy; TILs = tumor-infiltrating lymphocytes; PD-L1 = programmed death ligand 1; MRI = magnetic resonance imaging; BI-RADS = Breast Imaging, Reporting and Data System; LVI = lymphovascular invasion; DCIS = ductal carcinoma in situ; IHC = immunohistochemistry; FISH = fluorescence in situ hybridization; RECIST = Response Evaluation Criteria in Solid Tumors; SLNB = sentinel lymph node biopsy; LN = lymph node; pCR = pathological complete response; RCB = residual cancer burden; TNM = tumor-node-metastasis; ER = estrogen receptor; PR = progesterone receptor; HER2 = human epidermal growth factor receptor 2; LNR = lymph node ratio.