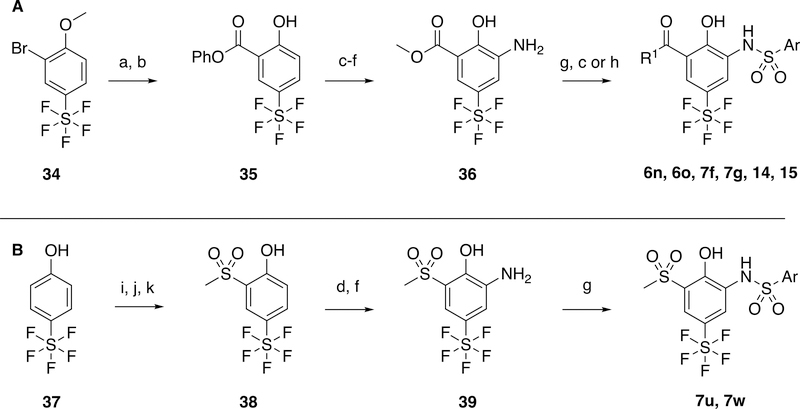
Scheme 3.
Synthesis of pentafluorosulfonyl products from commercial bromide intermediate, 30. Reagents and conditions: a) Phenyl formate, phenol, Pd(OAc)2, PtBu3.HBF4, Et3N, MeCN, 90 °C; b) BBr3, DCM, −78 °C; c) LiOH, THF, 65 °C; d) HNO3, H2SO4, DCM, 0 °C; e) H2SO4, MeOH, 65 °C; f) H2, Pd/C, MeOH, r.t.; g) ArSO2Cl, pyridine, DCM, r.t.; h) Amine, THF, 65 °C; i) Br2, FeCl3, AcOH, 0 °C; j) S2(CH3)2, Cu, pyridine, 115 °C, k) Oxone, EtOH, H2O, r.t.; k)