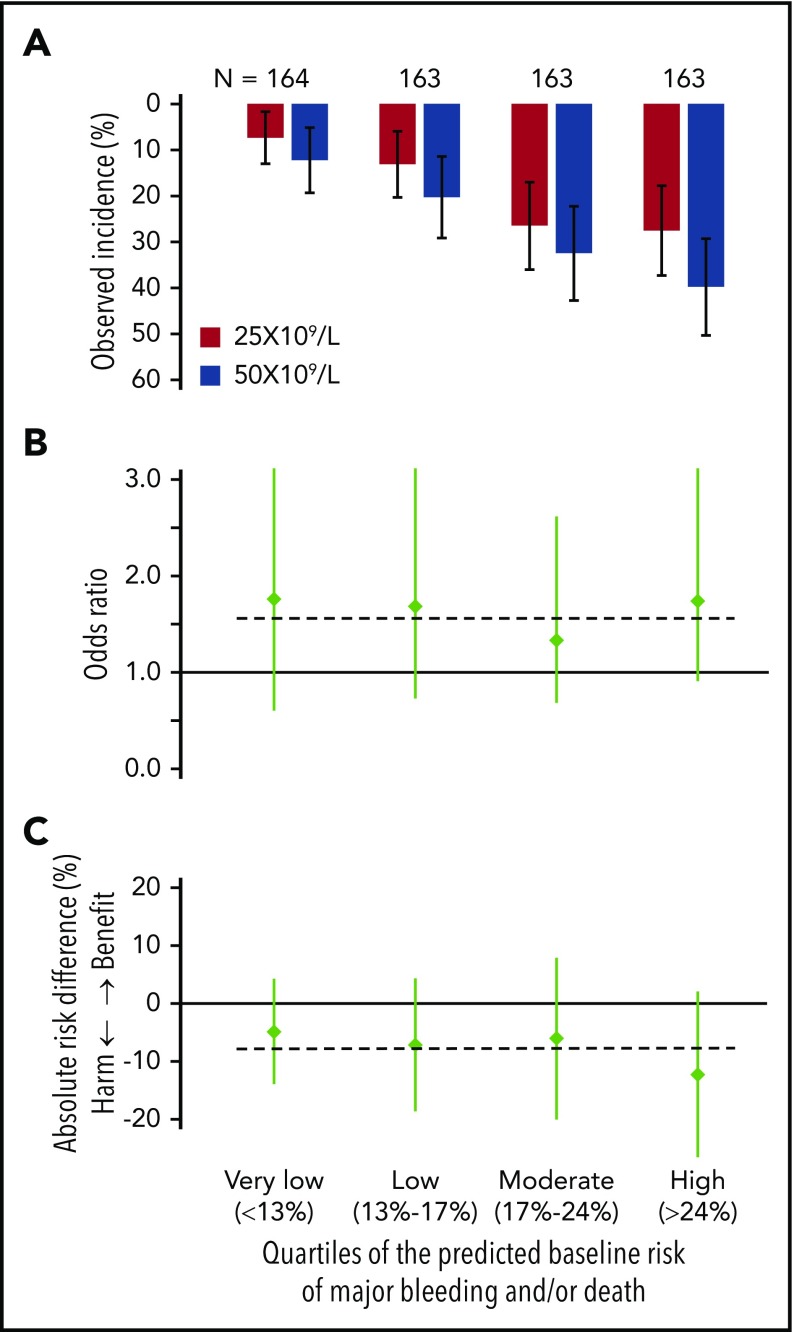
Figure 3.
Comparison of the occurrence of major bleeding and/or death within 28 days of randomization. The data of the 2 trial arms are expressed as observed incidence (A), odds ratios (B), and risk differences (C) for the included preterm neonates with severe thrombocytopenia in each of the 4 quartiles of their baseline risks (very low, <13%; low, 13%-16%; intermediate, 17%-24%; and high, >24%). Vertical lines represent 95% CI. Horizontal lines represent the previously published overall trial result, applicable to the nonexisting “average patient.” (A) In all 4 quartiles of baseline risk, the event rates of major bleeding and/or death among patients in the 25 × 109/L intervention arm were lower than the event rates in the 50 × 109/L intervention arm. (B) In all 4 quartiles of baseline risk, the ORs comparing patients in the 50 × 109/L intervention arm with those in the 25 × 109/L arm of the trial are similar. The horizontal line indicates the overall trial result (OR, 1.57). (C) In all 4 quartiles of baseline risk, the risk differences (incidence in the 25 × 109/L arm − incidence in the 50 × 109/L arm) and a horizontal line indicating the overall trial result (−7% absolute-risk difference). The absolute-risk difference indicated harm of the 50 × 109/L transfusion trigger in all 4 quartiles of baseline risk, which was less pronounced among patients with the very low baseline risk, compared with patients with the highest baseline risk.