Figure 5.
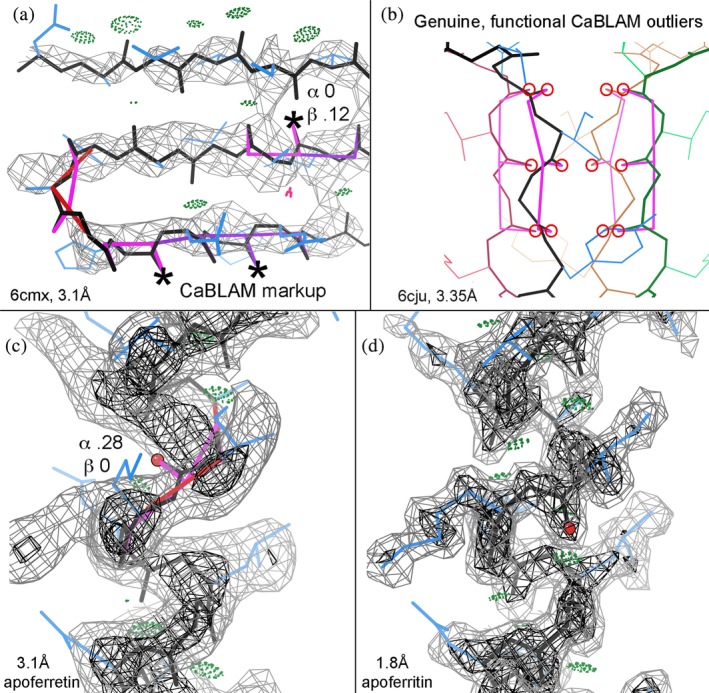
CaBLAM markup (Williams et al.4 in the 2018 Tools issue) is (at present) orthogonal to fitting/overfitting and suggests diagnosis of which misfit segments should be tried as regular α‐helix or β‐strand. (a) CaBLAM suggests regular β‐strands in http://firstglance.jmol.org/fg.htm?mol=6cmx at 3.1 Å,50 in spite of multiple successive outliers and almost no H‐bonds. Each starred CO just needs a near‐180° peptide rotation to achieve good β conformation and H‐bonding. (b) Shows genuine, functional CaBLAM outliers of extended strands forming the ion‐selectivity pore in 3.35 Å 6cju.51 (c) CaBLAM suggests regular α‐helix at a 0.28 score across a backward‐pointing CO outlier in the broad 3.1 Å apoferritin density, and (d) shows the CO correctly placed in its clear density at 1.8 Å
