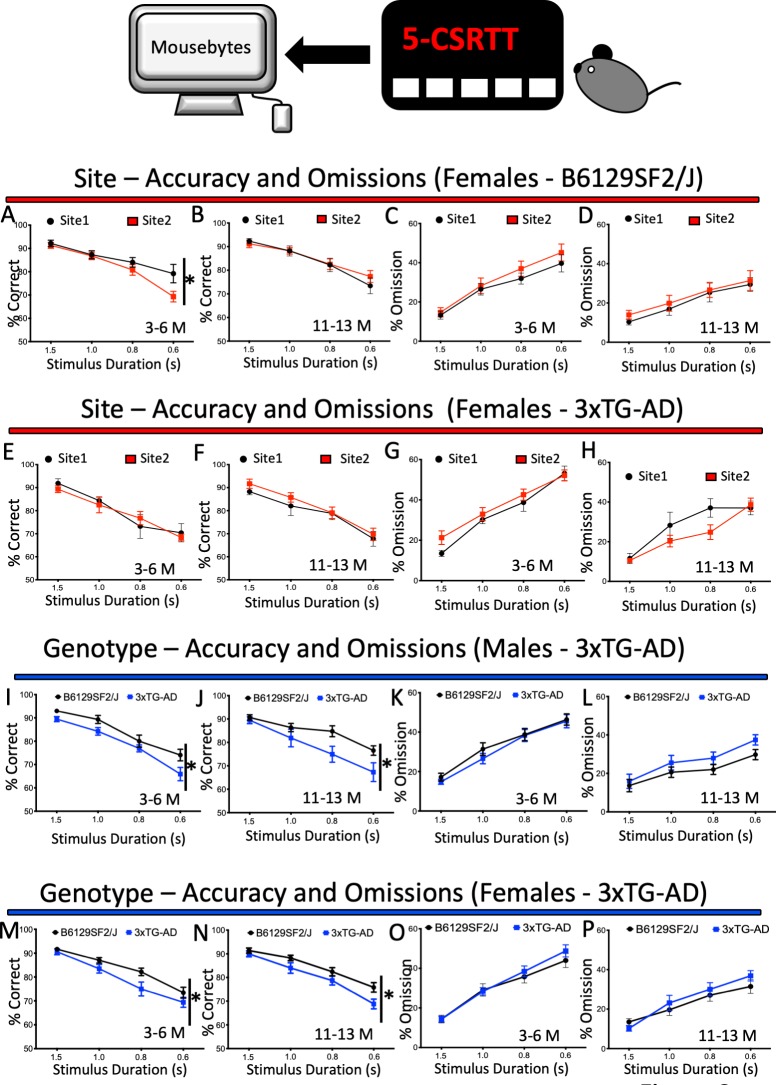
Figure 2. Performance and response measures of Male and Female mice during 5-CSRTT probe trials.
Mice were subjected to a series of probe trials and the averages of accuracy (% correct), omissions (%) and premature responses (number) were plotted at different ages. The plots were generated with data downloaded from MouseBytes and the links (datasets) for the individual analysis can be found in the results section. (A-D), longitudinal site comparison of the performance (accuracy and omissions) of female Wild-type controls (B6129SF2/J) at 3–6 and 11 to 13 months of age; (E-H) longitudinal site comparison of the performance (accuracy and omissions) of female 3xTG-AD at 3–6 and 11 to 13 months of age respectively; (I-L) comparison of the performance (accuracy and omissions) of 3xTG-AD male and their Wild-type controls (B6129SF2/J) at 3–6 and 11 to 13 months of age; (M-P) comparison of the performance (accuracy and omissions) of 3xTG-AD female mice and Wild-type controls (B6129SF2/J) at 3–6 and 11 to 13 months of age. Results are presented as means ± s.e.m.; data were analysed and compared using Repeated measure Two-Way ANOVA and Bonferroni multiple comparisons post-hoc test; *p<0.05, compared with control.
Figure 2—figure supplement 1. PDEBrd1 mutation effect in the behaviour of 5xFAD mice.

Figure 2—figure supplement 2. The effect of mild caloric restriction on Aβ(1–42) levels in male and female 3xTG and 5xFAD mice at 6 months of age.
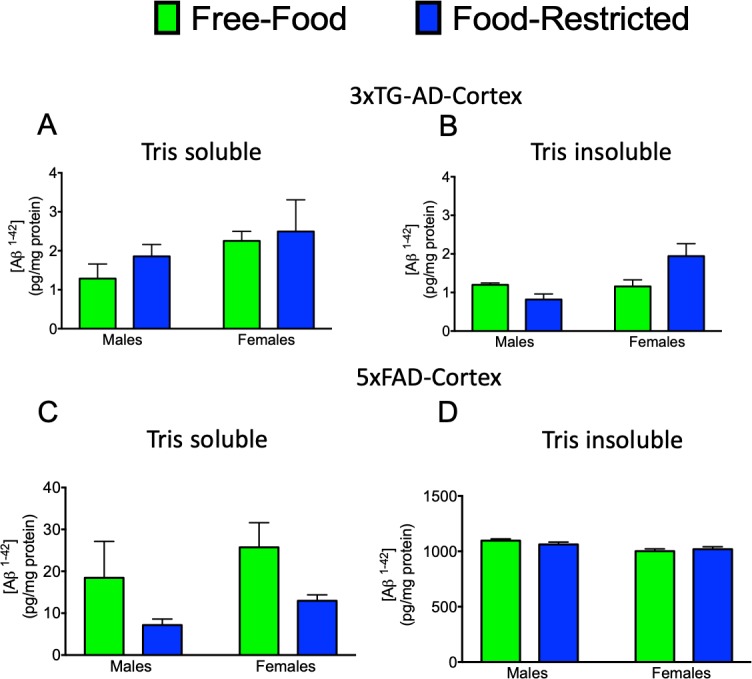
Figure 2—figure supplement 3. The effect of mild caloric restriction on amyloid pathology in male and female 5xFAD mice at 6 months of age.