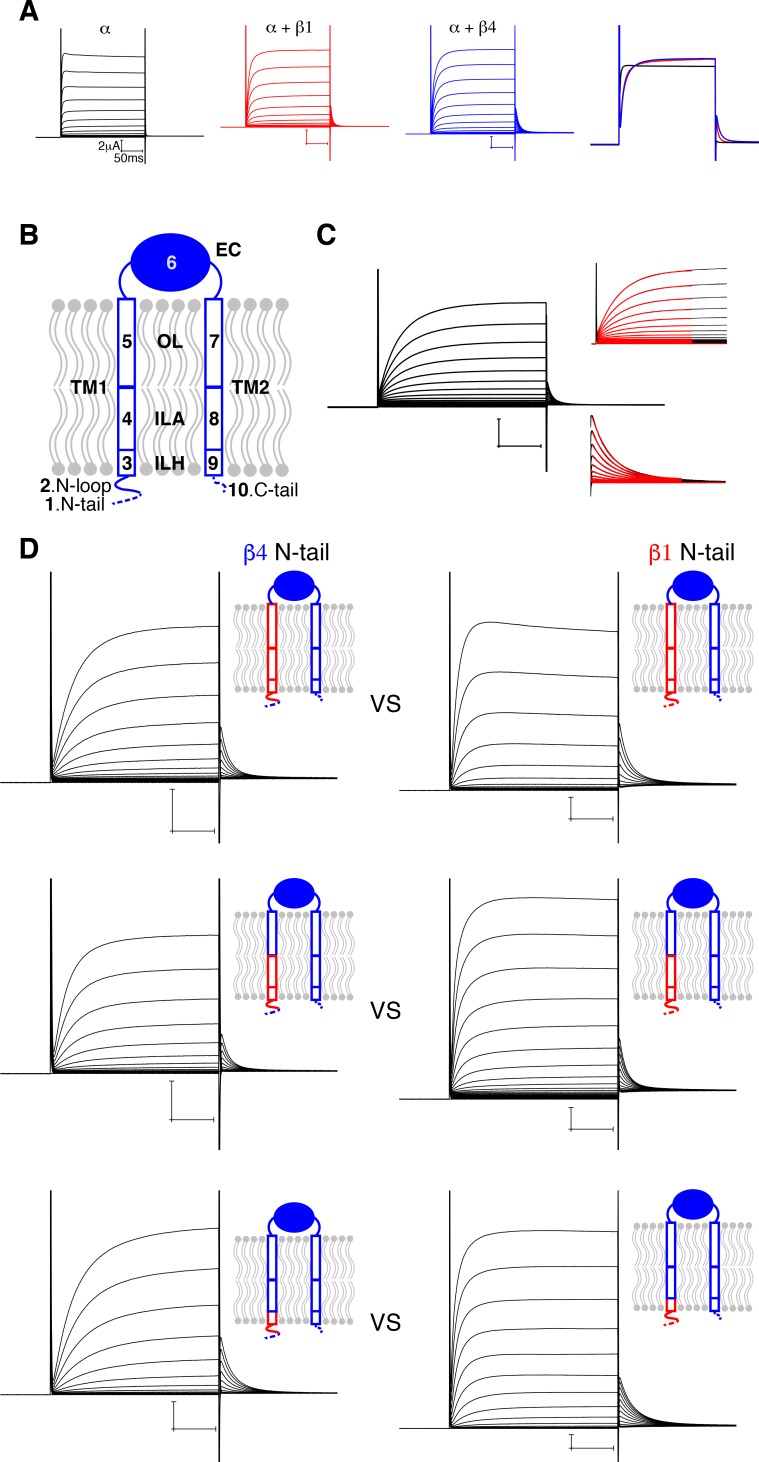
Figure 4. Influence of β4 N-terminus on Slo1 gating.
(A) Voltage-dependent channel activation of the human Slo1 channel alone and co-expressed with the β1 or β4 subunit. Representative current traces recorded using two-electrode voltage clamp (TEVC) are shown. Recording buffer: HEPES 5 mM, KCl 98 mM, CaCl2 0.3 mM, and MgCl2 1 mM. Voltage protocol: holding potential 0 mV, step from −80 to 120 mV in 10 mV incremental steps, step back to 40 mV. A superposition of one single sweep (stepping to 100 mV) from the left three recordings is shown on the right. (B) A schematic drawing of the β4 subunit showing the 10 regions divided based on the atomic structure. (C) Quantification of channel activation and deactivation kinetics by fitting with a single exponential function (see Materials and methods). (D) Comparison of three pairs of mutants demonstrated that the nature of N-tail is correlated with the activation kinetics: the presence of β4 N-tail (left column) results in slower activation kinetics than β1 N-tail (right column). Schematic drawing of each corresponding mutant is shown next to the representative current traces. Voltage protocols are the same as in panel (A).